石墨氮化碳/金纳米颗粒异质结构修饰的无创唾液尿酸检测电化学传感器
IF 4.9
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
通过在石墨氮化碳表面原位化学还原Au3+,合成了金纳米颗粒/石墨氮化碳异质结构纳米复合材料,并将其用于人唾液中尿酸(UA)的非侵入性电化学检测。在这种结构中,金纳米粒子(Au-NPs)作为高活性的电催化位点,而石墨化碳氮(g-C3N4)作为高表面积的支架,促进纳米粒子均匀分散和有效的电子转移。利用扫描电子显微镜(SEM)、高分辨率透射电子显微镜(HRTEM)、能量色散x射线光谱(EDX)、BET分析和红外光谱(IR)对Au-NPs进行了形态和元素表征,证实了锚定在g-C3N4薄片上的Au-NPs分布均匀。此外,通过电化学阻抗谱(EIS)和循环伏安法(CV)进行了电化学表征。电化学测量表明,Au-NPs@g-C3N4/CPE产生的UA氧化峰值电流明显高于裸CPE。在优化的pH条件、积累电位和差分脉冲参数下,传感器具有良好的线性校准范围0.5 ~ 10.0 μM (r = 0.9943),检出限为0.31 μM。人工唾液的选择性试验显示,在常见的唾液干扰素如抗坏血酸、肌酐和葡萄糖存在时,信号偏差可忽略不计(≤±2%)。使用实际唾液样品的峰值和回收率实验,回收率为95.56- 98.27%,证实了在复杂生物基质中具有较高的分析精度。此外,在环境储存60天后,电极保留了超过90%的初始响应,表明极好的稳定性。Au-NPs与g-C3N4的协同整合显著增强了催化活性、电子传递和UA吸附,使Au-NPs@g-C3N4/CPE成为一种经济、敏感、可靠的唾液点UA监测平台,用于临床诊断和健康应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
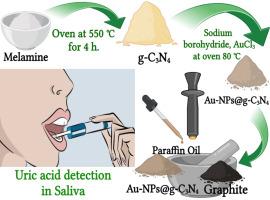
Electrochemical sensor modified with heterostructure of graphitic carbon nitride/gold nanoparticles for non-invasive uric acid detection in saliva
A gold nanoparticle/graphitic carbon nitride heterostructure nanocomposite was synthesized via an in-situ chemical reduction of Au3+ on the surface of graphitic carbon nitride and was applied for the non-invasive electrochemical detection of uric acid (UA) in human saliva. In this configuration, gold nanoparticles (Au-NPs) acted as highly active electrocatalytic sites, while graphitic carbon nitride (g-C3N4) served as a high-surface-area scaffold facilitating uniform nanoparticles dispersion and efficient electron transfer. Morphological and elemental characterization using scanning electron microscopy (SEM), High Resolution Transmission Electron Microscopy (HRTEM), energy-dispersive X-ray spectroscopy (EDX), BET analysis, and infrared spectroscopy (IR) confirmed the homogeneous distribution of Au-NPs anchored to the g-C3N4 sheets. Furthermore, electrochemical characterization was performed through electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). Electrochemical measurements demonstrated that Au-NPs@g-C3N4/CPE generated significantly higher UA oxidation peak currents compared with bare CPE. Under optimized pH conditions, accumulation potential, and differential pulse parameters the sensor exhibited a well-defined linear calibration range 0.5–10.0 μM (r = 0.9943) with a detection limit of 0.31 μM uric acid. Selectivity tests in artificial saliva showed negligible signal deviations (≤ ±2 %) in the presence of common salivary interferents such as ascorbic acid, creatinine, and glucose. Spike and recovery experiments using actual saliva samples achieved recoveries of 95.56-98.27 % confirming high analytical accuracy in complex biological matrices. Furthermore, the electrode retained over 90 % of its initial response after 60 days of ambient storage indicating excellent stability. The synergistic integration of Au-NPs with g-C3N4 significantly enhanced catalytic activity, electron transport, and UA adsorption making the Au-NPs@g-C3N4/CPE a cost-effective, sensitive, and reliable platform for point-of-care UA monitoring in saliva for clinical diagnostics and health applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensing and Bio-Sensing Research
Engineering-Electrical and Electronic Engineering
CiteScore
10.70
自引率
3.80%
发文量
68
审稿时长
87 days
期刊介绍:
Sensing and Bio-Sensing Research is an open access journal dedicated to the research, design, development, and application of bio-sensing and sensing technologies. The editors will accept research papers, reviews, field trials, and validation studies that are of significant relevance. These submissions should describe new concepts, enhance understanding of the field, or offer insights into the practical application, manufacturing, and commercialization of bio-sensing and sensing technologies.
The journal covers a wide range of topics, including sensing principles and mechanisms, new materials development for transducers and recognition components, fabrication technology, and various types of sensors such as optical, electrochemical, mass-sensitive, gas, biosensors, and more. It also includes environmental, process control, and biomedical applications, signal processing, chemometrics, optoelectronic, mechanical, thermal, and magnetic sensors, as well as interface electronics. Additionally, it covers sensor systems and applications, µTAS (Micro Total Analysis Systems), development of solid-state devices for transducing physical signals, and analytical devices incorporating biological materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


