沸石微球吸附、再生及固定化锶的合成及性能研究
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
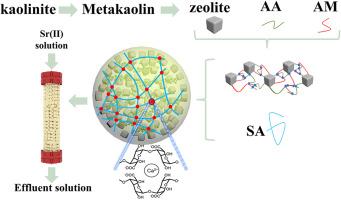
在这项研究中,成功制备了具有高多孔三维(3D)网络结构的沸石微球(ZMSs),具有高效的Sr2+吸附性能。这些ZMSs的合成使用了源自高岭石的4A沸石(Z4A),并通过海藻酸钠(SA)和Ca2+之间的交联作用促进了组装过程。沸石被固定在聚合物中,有利于吸附剂的吸附和解吸操作。静态吸附结果表明,在3 ~ 8的pH范围内吸附效果较好。Langmuir等温线拟合结果表明,最大饱和吸附量为51.6 mg/g。吸附量在1 h内可达到平衡吸附量的89.5%,具有较快的吸附动力学。柱测试证明了该材料的有效性能,668 mL含有100 mg/L Sr2+的流出溶液达到5%的突破点。以0.02 M EDTA-2Na为洗脱剂,5次循环后吸附剂的回收率可达58.73%。1100℃烧结ZMSs陶瓷在不同浸出液中的浸出率依次为:0.1 M HCl >0.1 M NaOH >0.1 M NaCl > H2O。研究结果表明,ZMSs具有吸附效率高、可操作性好、可回收性好、稳定性好等特点,是一种高效去除和处理放射性废水中Sr2+的新型吸附剂,适合大规模低浓度处理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and performance of zeolite microspheres for strontium adsorption, regeneration, and immobilization
In this study, zeolite microspheres (ZMSs) with a highly porous three-dimensional (3D) network structure exhibiting efficient Sr2+ adsorption properties were successfully prepared. The synthesis of these ZMSs involved the use of zeolite 4A (Z4A) derived from kaolinite, with the assembly process facilitated by the crosslinking interaction between sodium alginate (SA) and Ca2+. Zeolite was immobilized in polymers, which facilitated the adsorption and desorption operations of the adsorbent. Static adsorption results indicated that a favorable adsorption performance was achieved within the pH range of 3–8. The Langmuir isotherm fitting results revealed that the maximum saturated adsorption capacity was determined to be 51.6 mg/g. In addition, the adsorption capacity could reach 89.5 % of the equilibrium adsorption capacity within 1 h, demonstrating a relatively fast adsorption kinetics. Column tests demonstrated the effective performance of the material, with 668 mL of an effluent solution containing 100 mg/L Sr2+ reaching a 5 % breakthrough point. The recovery of the adsorbent can reach 58.73 % after five recycles when 0.02 M EDTA-2Na is used as the eluent. The leaching ratio of ZMSs ceramics sintered at 1100 °C in different leachates was found to follow the order: 0.1 M HCl >0.1 M NaOH >0.1 M NaCl > H2O. These findings showed ZMSs had high adsorption efficiency, convenient operability, recyclability and stability, serving as a novel adsorbent for efficient Sr2+ removal and disposal in radioactive wastewater, suitable for large-scale low-level treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


