纳米晶超细氧化铝的结晶相变
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
采用独特、经济、简单的沉淀法合成了超细氧化铝,并在750℃、950℃和1200℃的不同突出温度下煅烧,研究了氢氧化铝向α-氧化铝的相变行为。用x射线衍射(XRD)分析了氧化铝的α (α)、θ (θ)和γ (γ)相。采用Rietveld精细化方法对全粉模式拟合(WPPF)中不同相进行了定量分析。XRD谱图表明,在110℃时,氢氧化铝的贝汞石相较为突出。其中,在750℃、950℃和1200℃时,97.0%的bayerite相分别转变为γ相、95.0% θ (θ)相和100.0% α (α)相。傅里叶变换红外光谱(FTIR)显示,600 cm−1附近的波段属于氧化铝Al-O基团的拉伸模式。采用动态光散射(DLS)法测定了其流体动力直径和等电点(IEP),分别为282.3、252.9和209.1 nm, γ、θ和α氧化铝分别为7.0、4.7和5.0 nm。差示扫描量热法(DSC)和热重分析(TGA)的热分析表明,在250℃~ 1150℃范围内,由于氢氧化铝生成氧化铝,最终质量损失为21.08%。平均粒径约为42.0 ~ 49.0 nm,铝(Al)和氧(O)的原子质量分别为52.89%和47.03%,与透射电子显微镜(TEM)和能谱仪(EDS)所证实的理论值基本一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
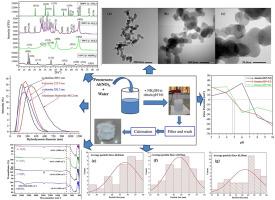
Crystallographic phase transformation of nanocrystalline ultrafine alumina
Ultrafine alumina was synthesized by a unique, cost-effective and simple precipitation method followed by calcination at different prominent temperatures at 750 °C, 950 °C and 1200 °C to investigate phase transformation behavior from aluminum hydroxide to α-alumina. Alpha (α), theta (θ) and gamma (γ) phases of alumina were identified by X-ray diffraction (XRD) analysis. Quantitative analysis of different phases was done by Rietveld Refinement in the whole powder pattern fitting (WPPF) method. The XRD pattern shows that initially at 110 °C bayerite phase of aluminum hydroxide is prominent. Where 97.0 % bayerite phase transferred to the γ phase, 95.0 % theta (θ) phase and 100.0 % alpha (α) phase at 750 °C, 950 °C and 1200 °C respectively. The bands around 600 cm−1 are assigned to the stretching mode of the Al–O group of alumina revealed by Fourier transform infrared spectroscopy (FTIR). The hydrodynamic diameter and isoelectric point (IEP) were determined using the dynamic light scattering (DLS) method which was 282.3, 252.9 and 209.1 nm and 7.0, 4.7 and 5.0 for γ, θ and α alumina respectively. The thermal analysis by differential scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA) shows that the final mass loss was 21.08 % observed over 250 °C–1150 °C due to the formation of alumina from aluminum hydroxide. The average particle size was around 42.0–49.0 nm and the atomic mass of elements aluminum (Al) = 52.89 % and oxygen (O) = 47.03 % which is almost similar to the theoretical value that is confirmed by transmission electron microscopy (TEM) coupled energy dispersive spectroscopy (EDS).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


