吡啶-腙基电荷转移探针用于检测水溶液中的Cu2+和Co2+离子
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-09-21
DOI:10.1016/j.saa.2025.126979
引用次数: 0
摘要
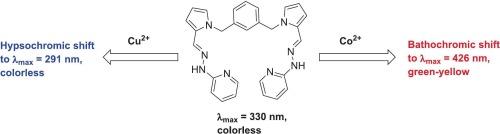
在环境监测领域,开发具有高灵敏度的选择性化学传感器来检测水介质中的过渡金属离子仍然存在相当大的挑战。在本研究中,我们制备并研究了1,3-二((2-((E)-(2-(吡啶-2-基)肼基)甲基)- 1h -吡啶-1-基)甲基)苯作为吡啶-腙基探针(受体a)选择性检测金属离子。探针与Cu2+和Co2+离子相互作用后,产生明显的光学变化,便于进行简单的离子检测。加入Cu2+溶液后,受体A的紫外可见吸收最大值发生了显著的色移至291 nm,而加入Co2+溶液后,受体A的紫外可见吸收最大值发生了显著的色移至426 nm。受体A能够选择性检测水溶液中的Cu2+和Co2+,不受其他金属离子的明显干扰,检出限分别为2.12 × 10−6 M和3.47 × 10−8 M,明显低于WHO标准。受体A对实际水样中Cu2+和Co2+的检测能力也得到了证实。这些发现突出了吡啶腙作为环境样品中Cu2+和Co2+离子实时监测的有效探针的潜在效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pyridine-hydrazone-based charge-transfer probe for detecting Cu2+ and Co2+ ions in aqueous solutions
In the field of environmental monitoring, there remain considerable challenges regarding the development of selective chemosensors with high sensitivity for detecting transition metal ions in aqueous media. In this study, we prepared and investigated 1,3-bis((2-((E)-(2-(pyridin-2-yl)hydrazineylidene)methyl)-1H-pyrrol-1-yl)methyl) benzene as a pyridine-hydrazone-based probe (receptor A) for the selective detection of metal ions. Upon interacting with Cu2+ and Co2+ ions, the probe responded with significant optical changes, thereby facilitating simple ion detection. The ultraviolet-visible absorption maximum of the receptor A underwent a remarkable hypsochromic shift to 291 nm upon the addition of Cu2+ solution, while the addition of Co2+ solution led to a bathochromic shift to 426 nm. Receptor A was capable of selectively detecting Cu2+ and Co2+ in aqueous solutions without any marked interference from other metal ions, with respective limits of detection of 2.12 × 10−6 and 3.47 × 10−8 M, which are significantly lower than the WHO guidelines. The ability of receptor A to detect Cu2+ and Co2+ in real water samples was also demonstrated. These findings highlight the potential utility of pyridine-hydrazones as an effective probe for real-time monitoring of Cu2+ and Co2+ ions in environmental samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


