3d打印功能化锶-丝素-羟基磷灰石支架通过免疫调节和顺序血管生成-成骨耦合促进骨再生
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
大骨缺损的修复仍然是一个重大的临床挑战。骨组织工程生物活性材料的开发为解决这些问题提供了有希望的解决方案。然而,缺乏血管化和内源性免疫排斥的风险严重阻碍了植入式生物材料在骨再生中的应用。因此,在本研究中,我们合成了一种多功能3d打印生物支架(EP@PCL/Sr),用于在免疫微环境下实现分阶段血管化骨再生,促进骨缺损修复。首先,EP@PCL/Sr支架的粗糙表面形态促进了细胞的增殖和粘附。此外,表没食子儿茶素-3-没食子酸酯,一种表面涂层成分,有助于免疫调节。最后,将锶丝素(Sr- sf)修饰的羟基磷灰石嵌入PCL支架中,释放Sr和Ca离子,促进血管生成和骨生成。体内和离体实验结果表明,EP@PCL/Sr支架具有良好的组织相容性、有效清除活性氧、强平衡免疫微环境和调节巨噬细胞极化、完美增强血管生成和促进成骨以促进骨再生等优异的多功能特性。此外,研究还揭示了EP@PCL/Sr支架通过激活ITGA10/PI3K/AKT通路促进BMSCs成骨的潜在机制。本研究为骨再生和骨缺损修复提供了一种综合性和创新性的策略,为其临床应用提供了新的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
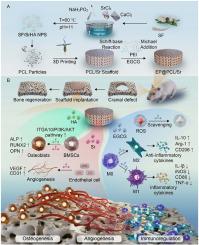
3D-printed functionalized strontium-silk fibroin-hydroxyapatite scaffolds facilitate bone regeneration via immunomodulatory and sequential angiogenic-osteogenic coupling
The repair of large bone defects remains a significant clinical challenge. The development of bioactive materials for bone tissue engineering offers promising solutions to address these problems. However, the lack of vascularization and the risk of endogenous immune rejection severely hinder the application of implantable biomaterials in bone regeneration. Therefore, in this study, we synthesized a multifunctional 3D-printed biological scaffold (EP@PCL/Sr) for achieving staged vascularized bone regeneration in the immune microenvironment to promote bone defect repair. Firstly, the rough surface morphology of the EP@PCL/Sr scaffolds enhanced cell proliferation and adhesion. Furthermore, epigallocatechin-3-gallate, a surface-coating component, contributed to immune regulation. Finally, strontium-silk fibroin (Sr-SF)-modified hydroxyapatite, embedded within the PCL scaffold, released Sr and Ca ions to improve both angiogenesis and osteogenesis. Both in vivo and ex vivo experimental results demonstrated that EP@PCL/Sr scaffolds exhibited excellent multifunctional properties, including good tissue compatibility, effective scavenging of reactive oxygen species, strong balancing of the immune microenvironment and regulation of macrophage polarization, perfect enhancement of angiogenesis and promotion of osteogenesis for promoting bone regeneration. Furthermore, the underlying mechanism were revealed that EP@PCL/Sr scaffolds promoted osteogenesis of BMSCs by activating the ITGA10/PI3K/AKT pathway. This study presents a comprehensive and innovative strategy for bone regeneration and bone defect repair, providing a new possibility for its clinical application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


