通过胺化途径实现甘油和乙二醇增值的多相催化剂
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从经济和环境的角度来看,将甘油作为生物柴油生产的副产品加工成有价值的化学品是一个有前途的领域。甘油增值的方法之一是对甘油及其衍生物(例如丙二醇)进行胺化,从而生产出各种具有高附加值的胺。氨以及伯胺和仲胺都可以作为胺化剂。本文综述了各种醇和甘油在非均相催化剂作用下的胺化反应。考虑了催化胺化过程的两种主要方法:在氢存在下的还原胺化和不引入额外氢的直接胺化。这些方法不仅在胺化过程机理上有所不同,而且在催化剂性质和工艺条件上也有所不同。传统的还原胺化催化剂是非贵金属过渡金属(Ni, Cu和Co),贵金属(Ru, Pd, Pt, Rh和Ir),或它们的组合沉积在多孔载体上。直接胺化催化剂的典型例子是沸石(例如,Y, ZSM-5和MOR)和多孔酸氧化物。在所有的情况下,考虑了还原和直接胺化过程的一般特征,并对具体的例子进行了系统化。总结了该领域存在的关键问题,并提出了成功实施催化胺化法甘油增值过程所需要完成的任务。本文章由计算机程序翻译,如有差异,请以英文原文为准。
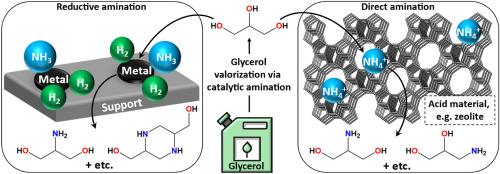
Heterogeneous catalysts for glycerol and glycol valorization via amination pathways
The processing of glycerol, as a by-product of biodiesel production, into valuable chemicals is a promising area from both economic and environmental perspectives. One of the approaches to glycerol valorization is the amination of glycerol and its derivatives (for example, propylene glycol), leading to the production of various amines with high added value. Ammonia, as well as primary and secondary amines can act as aminating agents. This review is devoted to the amination processes of various glycols and glycerol using heterogeneous catalysts.
Two main approaches to the catalytic amination process are considered: reductive amination in the presence of hydrogen and direct amination without the introduction of additional hydrogen. These approaches differ not only in the amination process mechanism but also in the nature of the catalyst and the process conditions. The traditional reductive amination catalysts are non-noble transition metals (Ni, Cu, and Co), noble metals (Ru, Pd, Pt, Rh, and Ir), or their combinations deposited on porous supports. Typical examples of direct amination catalysts are zeolites (e.g., Y, ZSM-5, and MOR) and porous acid oxides. For all cases, the general features of the processes of reductive and direct amination are considered, and specific examples are systematized. The key problems in this area are summarized, and the tasks necessary for the successful implementation of the valorization process of glycerol via the catalytic amination process are formulated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


