一种高效的g-C3N4@ZnO@ZrO2异质结构去除重金属离子-实验和计算模型
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
利用金属氧化物和碳基半导体的光降解和吸附已经成为从水生环境中去除有害有机染料和重金属离子的经济有效的方法。本文通过简单的超声法制备了一种新的CN@ZnO@ZrO2异质结构,以提高吸附Cd(II)离子的理化性能。x射线衍射(XRD)证实了六方g-C₃N₄相、六方ZnO相和单斜ZrO2相的形成。扫描电镜(SEM)和透射电镜(TEM)分析表明,g-C₃N₄纳米片上ZnO和ZrO2纳米颗粒分布均匀,能量色散x射线(EDX)分析证实了其元素纯度。该三元复合材料的比表面积为87.4 m2·g−1,孔体积为0.207 cm3·g−1,平均尺寸为14.53 nm。在最佳吸附条件下(温度25°C, pH: 7,吸附剂浓度为0.4 g·L−1,Cd2+浓度为60 mg·L−1),吸附时间为1400 min,吸附量为272.4 mg·g−1。动力学模拟和等温线分析表明,该过程符合拟二级动力学和Langmuir吸附模型。采用XRD、XPS、FTIR、SEM-EDS等方法对吸附机理进行了研究,密度泛函理论(DFT)计算提供了理论证实,与实验结果吻合良好。具体而言,DFT显示CN@ZnO@ZrO2(100)表面的Cd2+吸附能为- 3.84 eV,电荷转移量为1.16 |e|,支持了所观察到的优异吸附性能。总的来说,CN@ZnO@ZrO2异质结构在去除污染水中的污染物方面表现出了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
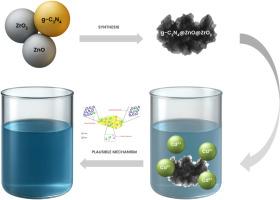
An efficient g-C3N4@ZnO@ZrO2 heterostructure for heavy metal ions removal – Experimental and computational modelling
Photodegradation and adsorption using metal oxide and carbon-based semiconductors have emerged as cost-effective and efficient methods for removing hazardous organic dyes and heavy metal ions from aquatic environments. In this work, a new CN@ZnO@ZrO2 heterostructure was successfully fabricated through a simple ultrasonication method to enhance physicochemical performance for Cd(II) ion adsorption. X-ray diffraction (XRD) confirmed the formation of hexagonal g-C₃N₄, hexagonal ZnO, and monoclinic ZrO2 phases. Scanning and transmission electron microscopy (SEM and TEM) showed a uniform distribution of ZnO and ZrO2 nanoparticles on the g-C₃N₄ nanosheets, while energy-dispersive X-ray (EDX) analysis confirmed the elemental purity. The ternary composite exhibited a relatively high specific surface area of 87.4 m2·g−1, with a pore volume of 0.207 cm3·g−1 and an average size of 14.53 nm. Adsorption experiments conducted under optimal conditions (1400 min at 25 °C, pH: 7, and 0.4 g·L−1 of adsorbent with 60 mg·L−1 of Cd2+ concentration) yielded a maximum adsorption capacity of 272.4 mg·g−1. kinetic modelling and isotherm analysis indicated that the process follows pseudo-second-order kinetics and Langmuir adsorption model, respectively. The adsorption mechanism was investigated using XRD, XPS, FTIR, and SEM-EDS mapping, while density functional theory (DFT) calculations provided theoretical confirmation, showing excellent agreement with the experimental results. Specifically, DFT revealed a a strongly negative Cd2+ adsorption energy of −3.84 eV and substantial charge transfer (1.16 |e|) on the CN@ZnO@ZrO2 (100) surface, supporting the observed superior uptake performance. Overall, the CN@ZnO@ZrO2 heterostructure demonstrated significant potential for use as an adsorbent in the removal of pollutants from contaminated water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


