基于CRP识别和新型传感技术应用的化疗心脏毒性风险评估新策略
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
癌症化疗虽然提高了患者的生存,但受到心脏毒性的限制。早期监测和风险评估至关重要。c反应蛋白(CRP)可以作为预测化疗期间心脏毒性的早期生物标志物,为开发新的心脏保护策略提供了重要机会。本研究将抗crp抗体负载在壳聚糖(CS)和功能化炭黑(f-CB)修饰的电极上。由于f-CB优良的导电性和CS的高通透性和强粘附性,我们成功构建了一种基于crp特异性免疫识别的化疗诱导心脏毒性早期监测和风险评估的免疫传感器。在最佳实验条件下,该免疫传感器在3.9 × 10−1 ~ 4 × 102 ng mL−1范围内具有良好的线性关系,检出限低至3.16 × 10−1 ng mL−1,灵敏度高于ELISA。该免疫传感器在化疗诱导的心脏毒性模型中检测CRP也表现出优异的选择性、重复性和稳定性,回收率为97.14%至107.65%。本研究为化疗引起的心脏毒性的早期检测和风险评估提供了一种高效、准确和实用的方法,从而支持治疗疗效和心脏安全性之间的平衡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
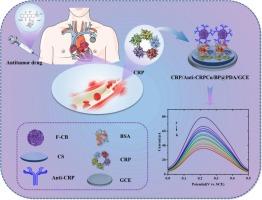
A new strategy for assessing chemo cardiotoxicity risk based on CRP recognition and the application of novel sensing technology
Cancer chemotherapy, while enhancing patient survival, is limited by cardiotoxicity. Early monitoring and risk assessment are essential. C-reactive protein (CRP) can serve as an early biomarker for predicting cardiotoxicity during chemotherapy, offering a critical opportunity to develop new cardioprotective strategies. This study used an anti-CRP antibody loaded onto an electrode modified with chitosan (CS) and functionalized carbon black (f-CB) as a target. Due to f-CB's excellent electrical conductivity and CS's high permeability and strong adhesion, an immunosensor for the early monitoring and risk assessment of chemotherapy-induced cardiotoxicity based on CRP-specific immunorecognition was successfully constructed. Under optimal experimental conditions, the immunosensor exhibited excellent linearity in the range of 3.9 × 10−1 to 4 × 102 ng mL−1 with a detection limit as low as 3.16 × 10−1 ng mL−1, making it more sensitive than ELISA. The immunosensor also demonstrated excellent selectivity, reproducibility, and stability in detecting CRP in a chemotherapy-induced cardiotoxicity model, with recovery rates ranging from 97.14 % to 107.65 %. This study presents an efficient, accurate, and practical approach for the early detection and risk assessment of chemotherapy-induced cardiotoxicity, thereby supporting a balance between therapeutic efficacy and cardiac safety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


