特定碳源驱动的代谢重编程调节了混合营养型细叶草的形态可塑性,促进了高价值代谢物的积累
IF 4.5
2区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
Algal Research-Biomass Biofuels and Bioproducts
Pub Date : 2025-09-19
DOI:10.1016/j.algal.2025.104308
引用次数: 0
摘要
微藻是一种很有前景的可持续生物资源,其代谢可塑性对环境适应和生物技术应用至关重要。然而,不同碳源调控微藻代谢网络和相关形态可塑性的确切机制仍未得到充分了解。本研究以细叶茅为模式生物。我们利用非靶向代谢组学技术,综合比较了光自养(PT)、乙醇-混合营养(MTE)和葡萄糖-混合营养(MTG)培养条件下的生长性能、形态特征、色素含量、paramylon积累和代谢谱。我们的研究结果揭示了薄叶菊显著的代谢可塑性。葡萄糖添加组的特定生长率和paramylon积累量最高,与PT组相比,体积paramylon产量提高了125.13%。细胞形态在培养过程中呈现动态变化;混合营养条件显著增加了细长细胞的比例,特别是在MTG组,其中细长细胞最终占92%。代谢组学分析表明,乙醇主要通过激活三羧酸循环和脂质代谢来刺激生长。相比之下,葡萄糖通过更直接的糖代谢途径、增强paramylon合成和更广泛的信号网络重建来增强生物量积累。脂质代谢的显著改变被确定为细胞形态动态转变的分子基础,从而证实了我们提出的“代谢-形态-功能”三维调控模型。本研究首次从代谢组学角度阐明了薄叶菊对不同碳源的适应性反应机制。该研究为研究微藻代谢可塑性提供了新的思路,并为优化培养策略提高副藻产量奠定了理论基础,对微藻生物技术的发展和应用具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
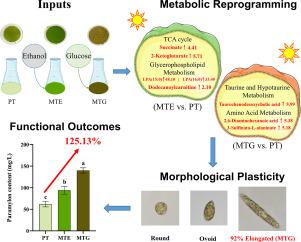
Metabolic reprogramming driven by specific carbon sources modulates morphological plasticity and enhances high-value metabolite accumulation in mixotrophic Euglena gracilis
Microalgae are recognized as promising sustainable bioresources, and their metabolic plasticity is pivotal for environmental adaptation and biotechnological applications. Nevertheless, the precise mechanisms through which distinct carbon sources regulate microalgal metabolic networks and the associated morphological plasticity remain inadequately understood. In this study, Euglena gracilis was employed as a model organism. We utilized untargeted metabolomics to comprehensively compare growth performance, morphological characteristics, pigment content, paramylon accumulation, and metabolic profiles under photoautotrophic (PT), ethanol-mixotrophic (MTE), and glucose-mixotrophic (MTG) cultivation conditions. Our findings reveal significant metabolic plasticity in E. gracilis. Glucose supplementation resulted in the highest specific growth rate and paramylon accumulation, with the volumetric paramylon yield increasing by 125.13 % compared to the PT group. Cell morphology exhibited dynamic alterations throughout the cultivation period; mixotrophic conditions markedly increased the proportion of elongated cells, particularly within the MTG group, where elongated cells ultimately represented 92 % of the population. Metabolomic analysis indicated that ethanol primarily stimulated growth via the activation of the tricarboxylic acid (TCA) cycle and lipid metabolism. In contrast, glucose enhanced biomass accumulation through more direct sugar metabolic pathways, augmented paramylon synthesis, and more extensive reconstruction of signaling networks. Significant alterations in lipid metabolism were identified as the molecular basis for the dynamic transitions in cell morphology, thereby corroborating our proposed “metabolism-morphology-function” three-dimensional regulatory model. This investigation provides the first metabolomic elucidation of the adaptive response mechanisms of E. gracilis to different carbon sources. It offers novel insights into microalgal metabolic plasticity and establishes a theoretical foundation for optimizing cultivation strategies to improve paramylon yield, holding considerable implications for the advancement and application of microalgal biotechnology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Algal Research-Biomass Biofuels and Bioproducts
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-
CiteScore
9.40
自引率
7.80%
发文量
332
期刊介绍:
Algal Research is an international phycology journal covering all areas of emerging technologies in algae biology, biomass production, cultivation, harvesting, extraction, bioproducts, biorefinery, engineering, and econometrics. Algae is defined to include cyanobacteria, microalgae, and protists and symbionts of interest in biotechnology. The journal publishes original research and reviews for the following scope: algal biology, including but not exclusive to: phylogeny, biodiversity, molecular traits, metabolic regulation, and genetic engineering, algal cultivation, e.g. phototrophic systems, heterotrophic systems, and mixotrophic systems, algal harvesting and extraction systems, biotechnology to convert algal biomass and components into biofuels and bioproducts, e.g., nutraceuticals, pharmaceuticals, animal feed, plastics, etc. algal products and their economic assessment
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


