碳负载Pt、Rh和SnO2纳米粒子的协同作用:微观结构对乙醇氧化选择性的影响
IF 4.6
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
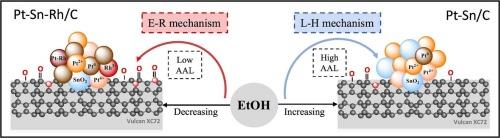
研究了碳负载Pt、Pt- sn、Pt- rh和Pt- sn - rh纳米颗粒在乙醇氧化反应(EOR)中的物理化学和电化学性能。x射线衍射(XRD)分析显示了Pt的面心立方晶体结构。透射电镜(TEM)图像显示,碳载体上的Pt- sn - rh纳米颗粒分散良好,平均粒径为2.8±0.2 nm。高分辨率TEM和能量色散x射线(EDX)显微分析证实了SnO2、Pt-Rh和Pt-Sn的存在。x射线光电子能谱(XPS)分析证实,Pt- sn - rh /C催化剂中存在金属Pt和SnO2。计时电流法结合加速降解试验(ADTs)表明Pt-Sn-Rh/C具有优异的催化稳定性。CO溶出伏安法还表明,Sn和Rh掺入Pt有利于CO在低电位下氧化。Pt-Sn- rh /C在低乙醇浓度下表现优异,这是由于Eley-Rideal机制,而Pt-Sn/C在高浓度下表现更好,这是由于富含sno2的表面有利于Langmuir-Hinshelwood途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergy of Pt, Rh and SnO2 nanoparticles supported on carbon: Influence of microstructures on the selectivity of ethanol oxidation
This study investigates the physicochemical and electrochemical properties of carbon-supported Pt, Pt-Sn, Pt-Rh, and Pt-Sn-Rh nanoparticles for ethanol oxidation reaction (EOR). X-ray diffraction (XRD) analysis reveals the face-centered cubic crystal structure of Pt. Transmission electron microscopy (TEM) images show well-dispersed nanoparticles on carbon support, with Pt-Sn-Rh exhibiting an average particle size of 2.8 ± 0.2 nm. High-resolution TEM and energy-dispersive X-ray (EDX) microanalysis confirm the presence of SnO2, Pt-Rh, and Pt-Sn. X-ray photoelectron spectroscopy (XPS) analysis confirms the presence of metallic Pt along with SnO2 in the Pt-Sn-Rh/C catalyst. Chronoamperometry combined with accelerated degradation tests (ADTs) demonstrates the excellent catalytic stability of Pt-Sn-Rh/C. CO stripping voltammetry also shows that the incorporation of Sn and Rh into Pt facilitates CO oxidation at low potentials. Pt-Sn-Rh/C excels at low ethanol concentrations due to the Eley-Rideal mechanism, whereas Pt-Sn/C performs better at high concentrations owing to SnO2-rich surfaces favoring the Langmuir-Hinshelwood pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Science and Engineering: B
工程技术-材料科学:综合
CiteScore
5.60
自引率
2.80%
发文量
481
审稿时长
3.5 months
期刊介绍:
The journal provides an international medium for the publication of theoretical and experimental studies and reviews related to the electronic, electrochemical, ionic, magnetic, optical, and biosensing properties of solid state materials in bulk, thin film and particulate forms. Papers dealing with synthesis, processing, characterization, structure, physical properties and computational aspects of nano-crystalline, crystalline, amorphous and glassy forms of ceramics, semiconductors, layered insertion compounds, low-dimensional compounds and systems, fast-ion conductors, polymers and dielectrics are viewed as suitable for publication. Articles focused on nano-structured aspects of these advanced solid-state materials will also be considered suitable.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


