原位FTIR确保苯硅烷安全淬火的应用
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
使用苯硅烷的工艺在多用途中试工厂中进行了扩展,并且需要彻底了解其水解释放可燃氢气的条件,以确保安全的加工和淬火程序。利用原位红外光谱、反应量热法和气体流量数据进行的研究表明,苯基硅烷的水解可以由少量的碱或金属催化,并且水解速率随溶剂的不同而变化很大。结果强调了在特定反应条件下对氢硅烷进行全面危害评价的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
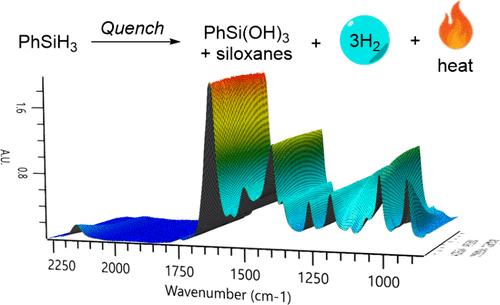
Use of In Situ FTIR to Ensure Safe Quench of Phenylsilane
A process using phenylsilane was scaled in a multipurpose pilot plant, and a thorough understanding of the conditions under which it hydrolyzes, releasing flammable hydrogen gas, was required to enable safe processing and quenching procedures. Studies utilizing in situ FTIR, reaction calorimetry, and gas flow data determined that the hydrolysis of phenylsilane can be catalyzed by a small amount of base or metal and the rate of hydrolysis can vary widely with the solvent. The results highlight the necessity for a full hazard evaluation under the specific reaction conditions when working with hydrosilanes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


