草酰乙酸盐感应促进对流感病毒感染的先天免疫抗病毒防御。
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
代谢途径决定细胞的命运和功能;然而,代谢物在宿主防御流感病毒中的确切作用仍不清楚。本研究采用药理学抑制和代谢组学分析表明,草酰乙酸(OAA)的代谢途径与流感病毒的抗病毒应答相结合。细胞质苹果酸脱氢酶1感知细胞内OAA进行二聚化,并作为支架招募转录因子ETS2,由激酶TAOK1在丝氨酸313处磷酸化。磷酸化的ETS2转运到细胞核中,支持TBK1的最佳表达,TBK1是I型干扰素反应不可或缺的激活因子。补充OAA可提供广谱抗病毒能力,而Acly基因消融引起的OAA缺乏可降低抗病毒免疫力,使小鼠更容易受到致命的H1N1病毒感染。我们的研究结果揭示了通过细胞OAA感应连接代谢和先天免疫协调防御病毒挑战的信号通路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
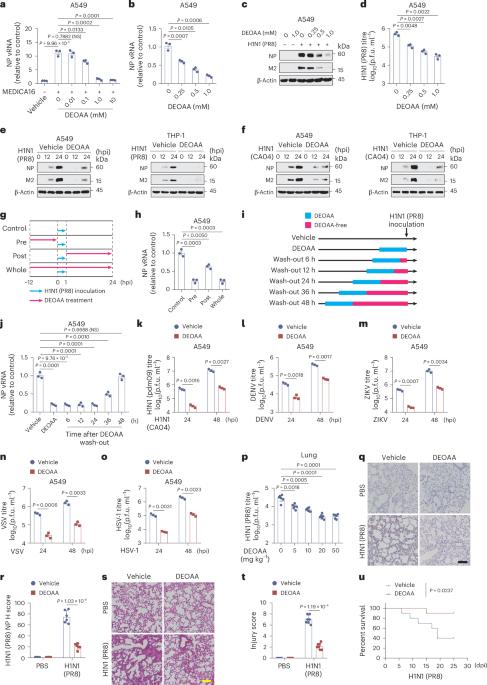
Oxaloacetate sensing promotes innate immune antiviral defence against influenza virus infection
Metabolic pathways determine cellular fate and function; however, the exact roles of metabolites in host defence against influenza virus remain undefined. Here we employed pharmacological inhibition and metabolomics analysis to show that the metabolic pathways of oxaloacetate (OAA) are integrated with antiviral responses to influenza virus. Cytosolic malate dehydrogenase 1 senses intracellular OAA to undergo dimerization and functions as a scaffold to recruit the transcription factor ETS2 for phosphorylation by the kinase TAOK1 at serine 313. The phosphorylated ETS2 translocates into the nucleus and supports optimal expression of TBK1, an indispensable activator of type I interferon responses. OAA supplementation provides a broad-spectrum antiviral ability, and OAA deficiency caused by Acly genetic ablation decreases antiviral immunity and renders mice more susceptible to lethal H1N1 virus infection. Our results uncover a signalling pathway through cellular OAA sensing that links metabolism and innate immunity to coordinate defence against viral challenge. Oxaloacetate is sensed by MDH1, which primes innate immune antiviral defence against influenza virus infection, thus resulting in the activation of ETS2 to promote TBK1-centred type I interferon signalling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


