发散催化:阐明配体控制的化学发散pd催化的分子内烯-硅环丁烷反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项工作中,基于我们之前发现的结构不同的膦配体可以有效地调节钯催化的分子内烯-硅环丁烷反应,从而产生两种不同的七元硅环产物,我们进一步证明了使用DPPF作为配体可以形成一种含有三种不同类型烯基的新型多取代硅烷化合物。这一发现大大扩展了使用相同金属催化剂和底物的发散催化的范围。通过实验和计算相结合的研究,我们全面阐明了反应机理,该反应是由硅碳键激活硅环丁烷部分,然后插入烯形成一个七元palladacycle作为关键中间体。随后的反应途径由于配体依赖的还原性消除与β-H消除过程的调制而发生分歧。此外,硅环丁烷部分的开环中间体可以通过还原消除或钯中间体的1,3迁移位移进行额外的发散途径,从而产生三种不同类型的基于发散催化转化的产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
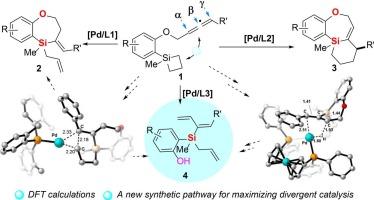
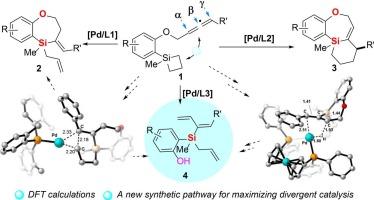
Divergent catalysis: elucidation of ligand-controlled chemo-divergent Pd-catalyzed intramolecular allene-silacyclobutane reactions
In this work, building upon our previous discovery that structurally distinct phosphine ligands can effectively modulate palladium-catalyzed intramolecular allene-silacyclobutane reactions to afford two different seven-membered silacyclic products, herein we further demonstrated that using DPPF as ligand leads to the formation of a novel polysubstituted silane compounds containing three distinct types of alkenyl groups. This finding significantly expands the scope of divergent catalysis using identical metal catalyst and substrate. Through combined experimental and computational studies, we have comprehensively elucidated the reaction mechanism, in which the reaction is initiated by silicon-carbon bond activation of the silacyclobutane moiety, followed by allene insertion to form a seven-membered palladacycle as the key intermediate. The subsequent reaction pathways diverge due to the ligand-dependent modulation of reductive elimination versus β-H elimination processes. Furthermore, the ring-open intermediates of silacyclobutane moiety can undergo additional divergent pathways through either reductive elimination or 1,3-migration shift of the palladium intermediate, resulting in three distinct types of products for this divergent catalysis-based transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


