含水锌/二氧化锰电池的空间和化学异质性:含锌和含锰配合物的作用
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
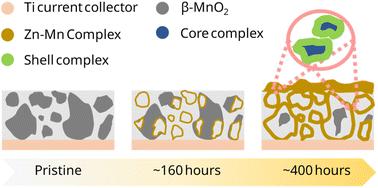
由于其环境安全和低成本的特性,锌/二氧化锰水电池是一种有前途的、安全的电网规模储能替代方案。在温和的pH水溶液中,溶解-沉积反应机制由于其与电池设计的相关性,最近获得了重要意义。了解具体位置和反应过程对高效电池至关重要。研究表明,在放电过程中,羟基硫酸锌(zs)主要在溶解的MnO2颗粒附近形成。在随后的循环中作为电荷反应物的宿主,使用operando x射线荧光显微镜观察电荷产物的形态。经过~ 400小时的循环后,容量衰减与锌-锰核-壳相的形成有关,这归因于电极中不可逆的核相,通过三维化学映射可视化。总的来说,这项研究强调了在设计先进的电网规模储能技术的电极和化学物质时,理解局部形态进化的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatial and chemical heterogeneity in aqueous Zn/MnO2 batteries: role of Zn and Mn containing complexes
Aqueous Zn/MnO2 batteries are a promising, safe alternative for grid-scale energy storage, owing to their environmentally safe and low-cost nature. The dissolution–deposition reaction mechanism in a mild aqueous pH regime has recently gained significance due to its relevance in battery design. Comprehending both the specific locations and the way reaction progresses is crucial for efficient batteries. This study demonstrates that Zinc Hydroxy Sulfate (ZHS) formed during discharge primarily near the dissolved MnO2 particles. Acting as a host for charge reactants in subsequent cycles, the charge product morphology was visualized using operando X-ray fluorescence microscopy. After ∼400 hours of cycling, capacity fade was linked to the formation of a Zn–Mn core–shell phase which is attributed to an irreversible core phase in the electrode, visualized through three-dimensional chemical mapping. Overall, this research underscores the importance of understanding local morphological evolution in designing electrodes and chemistries for advanced grid-scale energy storage technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


