二磷酸基水凝胶微球用于靶向经动脉放射栓塞和化疗栓塞治疗
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
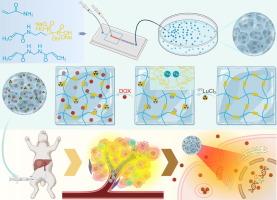
经动脉放射栓塞(TARE)是一种非常有效的治疗不可切除的肝细胞癌(HCC)的方法。然而,目前临床上可用的放射性90Y树脂微球面临同位素泄漏和低比活度等重大挑战,影响了安全性和治疗效果。为了解决这些挑战,我们提出了一种新的基于二膦酸盐的水凝胶微球(dpm),用于更安全、更有效的TARE治疗。二膦酸盐与各种金属离子具有很强的螯合能力,这为用不同的治疗性金属核素紧密标记水凝胶微球提供了一种通用策略。方法采用微流控技术制备dms,并进行177Lu放射性标记和DOX负载。在体外和原位肝癌兔模型中系统评估了其理化特性、放射稳定性和双模治疗效果,并通过组织病理学和血清生化验证了其生物安全性。结果dpm在15 min内的标记效率为98.3% %。同时,在10 %的胎牛血清溶液中,放射性标记率在7 天内保持99 %,表现出优异的放射性稳定性。高的体外放射性稳定性也与体内实验相一致。此外,dpm的多孔结构可以实现化疗药物的高负载和控释,使水凝胶微球成为经导管动脉化疗栓塞(TACE)的理想载体。通过TARE和TACE的结合,我们开发了一种新的177Lu-DPMs@DOX平台,对HCC具有显著的治疗效果,没有任何观察到的副作用。本研究介绍了一种突破性的方法,使用高效的二膦酸盐微球为不可切除的HCC和其他具有挑战性的肿瘤提供精确、安全、有效的治疗本文章由计算机程序翻译,如有差异,请以英文原文为准。
Disphosphate based hydrogel microspheres for targeted transarterial radioembolization and chemoembolization therapies
Introduction
Transarterial radioembolization (TARE) is a highly effective treatment for unresectable hepatocellular carcinoma (HCC). However, current clinically available radioactive 90Y resin microspheres face significant challenges such as isotope leakage and low specific activity, compromising the safety and therapeutic efficacy.Objectives
To address these challenges, we propose a novel diphosphonate based hydrogel microspheres (DPMs) for safer and more effective TARE therapies. The diphosphonate have a strong chelating capability with various metal ions which provide a universal strategy for tightly labelling the hydrogel microsphere with different therapeutic metal nuclides.Methods
In this study, DPMs were fabricated via microfluidic technology, followed by 177Lu radiolabeling and doxorubicin (DOX) loading. Physicochemical characterization, radiostability assays, and dual-modality therapeutic efficacy were systematically evaluated in vitro and in orthotopic HCC rabbit models, with biosafety validated through histopathology and serum biochemistry.Results
DPMs achieved a labeling efficiency of 98.3 % within 15 min. Meanwhile, the radiolabeling rate maintained 99 % over 7 days in a 10 % fetal bovine serum solution, demonstrating exceptional radiostabilty. The high in vitro radiostability is also consistent with in vivo experiments. Additionally, the porous structure of DPMs enables high loading and controlled release of chemotherapeutic drugs, making the hydrogel microspheres also the ideal carriers for transcatheter arterial chemoembolization (TACE). By combining TARE and TACE, we have developed a novel 177Lu-DPMs@DOX platform, exhibiting remarkable therapeutic performance against HCC without any observed side effect.Conclusion
This study introduces a groundbreaking approach using highly effective diphosphonate based microspheres to deliver precise, safe, and powerful treatment for unresectable HCC and other challenging tumors求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


