使用固定位置光学镊子的无检测高通量细胞分选
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
基于光学镊子的分选是一种很有前途的技术,可以以最小的损伤分选细胞,提供温和的非接触辐射力,以精确地操纵细胞进入收集通道。然而,现有的基于光镊子的分选系统严重依赖于检测技术来对细胞类型进行分类,并且需要不断地重新定位光镊子以将细胞引导到收集通道,这限制了吞吐量并增加了系统的复杂性。在这项工作中,我们提出了一种使用固定位置光镊的无检测、高通量细胞分选方法。具体而言,我们设计了一种具有弧形凸起的微流控芯片,使固定位置的光镊能够通过水动力和光力的共同作用,有效地将目标细胞引导到收集通道中。这种方法显著提高了吞吐量,达到10个单元/秒的理论吞吐量,同时降低了系统复杂性和成本。通过分选不同大小的颗粒,纯度为98.2%,从红细胞中分离癌细胞,纯度为95.5%,验证了该系统的性能。同时,该系统保持高细胞活力,使其非常适合应用于临床诊断、药物筛选和单细胞基因组学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
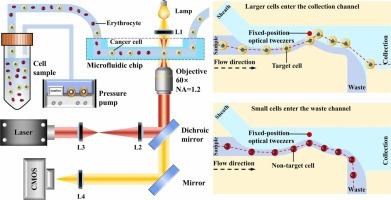
Detection-free high-throughput cell sorting using fixed-position optical tweezers
Optical tweezers-based sorting is a promising technique for sorting cells with minimal damage, offering a gentle, non-contact radiation force to precisely manipulate cells into a collection channel. However, existing optical tweezers-based sorting systems heavily rely on detection technologies to classify cell types and require continuous repositioning the optical tweezers to steer cells toward the collection channel, which limits throughput and increases system complexity. In this work, we present a detection-free, high-throughput cell sorting method using fixed-position optical tweezers. Specifically, we designed a microfluidic chip featuring an arc-shaped protrusion, allowing the fixed-position optical tweezers to efficiently direct target cells into the collection channel through the combined influence of hydrodynamic forces and optical forces. This approach markedly enhances throughput, reaching a theoretical throughput of 10 cells/s, while simultaneously reducing system complexity and cost. The performance of the system was validated by sorting particles of varying sizes with a purity of 98.2 % and by separating cancer cells from erythrocytes with a purity of 95.5 %. Meanwhile, the system maintains high cell viability, making it well-suited for applications in clinical diagnostics, drug screening, and single-cell genomics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


