氮掺杂碳纳米管封装nZVI作为纳米约束催化剂的原位生长,减轻了类芬顿催化中铁阳离子浸出和低氧化剂利用率的障碍
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
纳米级零价铁基高级氧化工艺(nZVI-AOPs)是治理新兴污染物最强大的技术之一,但它容易受到大量溶解铁的浸出问题的影响,这些铁本质上具有较低的芬顿反应性,导致不良的二次污染和氧化剂的无用消耗。本文提出了一种螯合辅助共组装纳米约束策略,在三聚氰胺和双齿n配体1,10-菲罗啉的辅助下,实现了含铁掺杂氮纳米管的自组装。得益于独特的纳米管结构、均匀的N掺杂和良好封装的nZVI的协同作用,获得的Fe0@NCNTs-2在激活过氧单硫酸盐(PMS)以消除和矿化双酚A (BPA)方面表现出良好的效率。豆荚状核壳有效地保护了内部活性位点,减少了各种降解过程中金属离子的泄漏。纳米结构赋予Fe0@NCNTs-2对PMS的多位点吸附能力,同时将氧化剂的利用效率提高了50% %以上。实验结果和密度泛函理论(DFT)计算共同揭示了纳米约束下nzvi介导的电子金属-载体相互作用(EMSI)机制是导致PMS在Fe0@NCNTs表面吸附能较低和无效消耗的原因,并大大加快了电子转移。同时还触发了非自由基主导(1O2和电子转移)氧化机制,揭示了纳米限制催化剂在复杂水基质中具有宽ph取向和优异稳定性的原因。纳米约束策略的引入有利于促进nZVI-AOPs的蓬勃发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
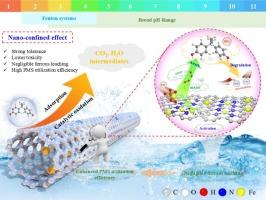
In situ growth of N-doped carbon nanotubes encapsulated nZVI as nanoconfinement catalyst for mitigating the hurdles of ferric cations leaching and low oxidant utilization in Fenton-like catalysis
The nanoscale zero-valent iron-based advanced oxidation processes (nZVI-AOPs) exemplify one of the most robust technologies for governing the emerging contaminants but are susceptible to the leaching issue of substantial dissolved iron species with intrinsically low Fenton reactivity, resulting in undesirable secondary pollution and the futile consumption of oxidants. Here, a chelation-assisted co-assembly nanoconfinement tactic was developed to achieve the self-assembly of nitrogen-doped carbon nanotubes embedded with Fe species, assisted by the melamine and bidentate N-ligand 1,10-Phenanthroline. Benefiting from the synergy of distinctive nanotube structure, uniform N doping, and well-encapsulated nZVI, the as-obtained Fe0@NCNTs-2 manifested decent efficiency for activating peroxymonosulfate (PMS) to eliminate and mineralize bisphenol A (BPA). The pod-like core-shell efficaciously protected the inner active sites and minimized the leakage of metal ions under various degradation processes. The nanoconfined structure endows Fe0@NCNTs-2 with a multi-site adsorption of PMS, along with enhancing the utilization efficiency of oxidants by over 50 %. Experimental results and density functional theory (DFT) calculations jointly divulged that the nanoconfined nZVI-mediated electronic metal-support interactions (EMSI) mechanism under the nanoconfinement was responsible for the lower adsorption energy and futile consumption of PMS on the Fe0@NCNTs surface and considerably hastened electron transfer, along with triggering the nonradical-dominated (1O2 and electron-transfer) oxidation mechanism and unraveling the reason for the wide-pH orientation and exceptional stability of nanoconfined catalysts in the complicated water matrix. The introduction of the nano-confinement strategy is conducive to promoting the vigorous development of nZVI-AOPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


