吲哚环与TMSCCl3/TMSCBr3扩展成喹啉
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
反应性适应物种形成将反应物视为一个动态的反应单元供应池,为不同的反应模式提供机会性反应匹配的多种途径,揭示了吲哚- tmsccl3 /TMSCBr3反应的C2C3-CCl /CBr插入环扩展成喹啉,N1-H-TMS亲核取代和N1-H-CCl3亲核取代。本文章由计算机程序翻译,如有差异,请以英文原文为准。
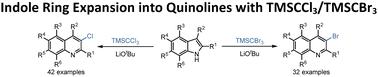
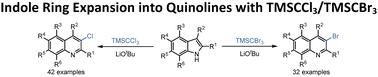
Indole ring expansion into quinolines with TMSCCl3/TMSCBr3
Reactivity adaptation speciation perceives reactants as a dynamic supply pool of shifting reactive units for opportunistic reactivity-matching diverse-manifold pathways to varied reactivity patterns, revealing the title C2C3–CCl/CBr insertion ring expansion of indole into quinoline, N1–H–TMS nucleophilic substitution, and N1–H–CCl3 nucleophilic substitution for the indole–TMSCCl3/TMSCBr3 reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


