论文征集:大周期作为具有挑战性的药物靶点的治疗方式
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
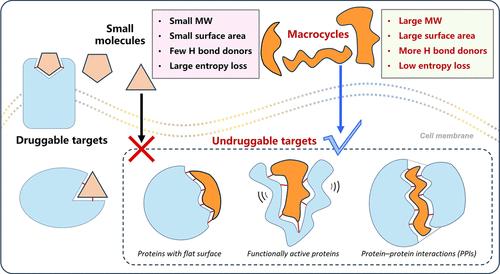
构成可行治疗剂的概念已经大大扩展,从传统的小分子转向更复杂的结构。其中,大环化合物由于其独特的调节具有挑战性的生物靶点的能力而成为有前途的一类。与传统的小分子不同,大环具有中等分子量,构象约束和扩展的表面积,能够与平面蛋白质表面和蛋白质-蛋白质相互作用界面进行高亲和力结合,这些目标通常被认为是“不可药物”的。值得注意的是,在与人类疾病相关的靶蛋白中,高达85%仍被认为是不可药物的。(1)大环提供了适应性、选择性和改进的药理学特征的令人信服的组合。(2)它们的环状结构降低了结合时的构象熵,增强了靶结合,增加了结合亲和度。这些特征使它们在干扰复杂的生物分子相互作用方面特别有价值,这些相互作用是疾病机制的核心,但对于典型的小分子或生物制剂仍然难以捉摸(图1)。近年来,大环化合物在合成策略、计算设计和生物学评价方面取得了重大进展。Hiroaki Suga, (3) Christian Heinis, (4) Herbert Waldmann, (5) Felix Hausch, (6) Jan Kihlberg, (7) Mate Erdelyi,(2)丁克,(8)李洪林,(9)陆晓云,(10)we(11)等人为推进大环类药物化学的研究做出了贡献。值得注意的是,通过创新机制,包括诱导新形态相互作用的分子胶策略,大环已显示出靶向致癌蛋白(如KRAS)、转录因子和各种调节复合物的前景。此外,大环支架与肽、(3,11)类肽或合成化学物质的整合,为开发具有更高稳定性、膜渗透性和口服生物利用度的化合物开辟了广阔的前景。尽管取得了这些进展,大环疗法的发展仍然面临着相当大的挑战。合成可达性、结构多样化和预测建模等方面的限制阻碍了化学空间的有效开发。目前迫切需要新的大环化方法、高效的筛选平台和优化大环化合物类药物性质的策略。本期《药物化学杂志》特刊致力于“大环作为具有挑战性的药物靶点的治疗方式”,邀请关注大环作为治疗剂在设计、合成和应用方面的最新创新。感兴趣的主题包括但不限于新的大环化反应和策略大环化合物的计算设计和建模大环肽和拟肽物涉及大环的靶向蛋白质降解和分子胶蛋白-蛋白质相互作用的大环抑制剂改善细胞渗透性和口服生物利用度的策略针对不可药物蛋白的案例研究(例如RAS, c-MYC,大环文库筛选技术的进展大环-靶标相互作用的结构研究和机制见解特刊的提交截止日期为2026年5月31日。文章将在接受后以滚动的方式在定期刊物上发表,然后在专门的在线文集中汇集。《药物化学杂志》欢迎原创研究文章、观点和药物注释。我们期待在截止日期前收到您的投稿。投稿前请参考作者指南。如有任何关于投稿的问题,请发送至jmc@jmedchem.acs.org。本文引用了11个其他出版物。本文档已更新,点击查看更多信息。这篇文章尚未被其他出版物引用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Call for Papers: Macrocycles as Therapeutic Modalities for Challenging Drug Targets
The perception of what constitutes a viable therapeutic agent has expanded considerably, moving beyond traditional small molecules to embrace more complex architectures. Among these, macrocyclic compounds have emerged as a promising class due to their unique ability to modulate challenging biological targets. Unlike conventional small molecules, macrocycles possess intermediate molecular weights, conformational restraint, and extended surface areas that enable high-affinity binding to flat protein surfaces and protein–protein interaction interfaces─targets often deemed “undruggable”. Notably, among human disease-related target proteins, up to 85% are still considered undruggable. (1) Macrocycles offer a compelling combination of adaptability, selectivity, and improved pharmacological profiles. (2) Their cyclic structures reduce conformational entropy upon binding, enhancing target engagement and increasing binding affinity. These characteristics make them particularly valuable for interfering with intricate biomolecular interactions that are central to disease mechanisms but remain elusive to typical small molecules or biologics (Figure 1). Recent years have witnessed significant advances in synthetic strategies, computational design, and biological evaluation of macrocyclic compounds. Hiroaki Suga, (3) Christian Heinis, (4) Herbert Waldmann, (5) Felix Hausch, (6) Jan Kihlberg, (7) Mate Erdelyi, (2) Ke Ding, (8) Honglin Li, (9) Xiaoyun Lu, (10) we (11) and others have contributed to advancing research on the medicinal chemistry of macrocycles. Notably, macrocycles have shown promise in targeting oncogenic proteins such as KRAS, transcription factors, and various regulatory complexes through innovative mechanisms, including molecular glue strategies that induce neomorphic interactions. Furthermore, the integration of macrocyclic scaffolds with peptide, (3,11) peptidomimetic, or synthetic chemistries has broadened the horizon for developing compounds with enhanced stability, membrane permeability, and oral bioavailability. Despite these advances, the development of macrocyclic therapeutics continues to face considerable challenges. Limitations in synthetic accessibility, structural diversification, and predictive modeling hinder the efficient exploration of chemical space. There is a pressing need for novel macrocyclization methodologies, efficient screening platforms, and strategies to optimize the drug-like properties of macrocyclic compounds. This Special Issue of the Journal of Medicinal Chemistry, dedicated to “Macrocycles as Therapeutic Modalities for Challenging Drug Targets”, invites contributions that highlight recent innovations in the design, synthesis, and application of macrocycles as therapeutic agents. Topics of interest include, but are not limited to Novel macrocyclization reactions and strategies Computational design and modeling of macrocyclic compounds Macrocyclic peptides and peptidomimetics Targeted protein degradation and molecular glues involving macrocycles Macrocyclic inhibitors of protein–protein interactions Strategies to improve cell permeability and oral bioavailability Case studies targeting undruggable proteins (e.g., RAS, c-MYC, transcription factors) Advances in screening technologies for macrocyclic libraries Structural studies and mechanistic insights into macrocycle-target interactions The submission deadline for the Special Issue is May 31, 2026. Articles will be published on a rolling basis in regular issues upon acceptance and later assembled in a dedicated online collection. The Journal of Medicinal Chemistry welcomes original research Articles, Perspectives, and Drug Annotations. We look forward to receiving your submissions by the deadline. Please consult the Author Guidelines before submission of the manuscript. For any questions regarding submission into this Special Issue, they may be sent to jmc@jmedchem.acs.org. This article references 11 other publications. This document has been updated Click for further information. This article has not yet been cited by other publications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


