固态电化学能量转换与存储装置的发展趋势
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
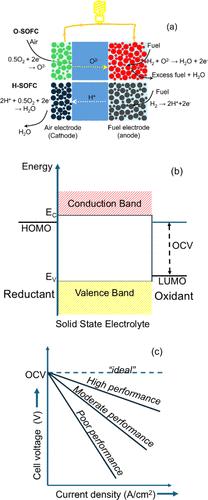
作为ACS能源通讯特刊“能源研究的演变景观:编辑组的观点”的一部分发表。图1所示。(a)固体氧化物燃料电池(O-SOFC)和陶瓷质子导电H-SOFC的工作原理示意图。与SOFC不同,质子传导燃料电池中没有燃料稀释,因为水是在氧电极(阴极)产生的。(b)电化学电池中具有建议/期望的氧化剂和还原剂能级的热力学稳定电池示意图。(c)陶瓷燃料电池“理想”、“高”、“中等”和“差”性能的典型极化曲线示意图。在中温区(500-750℃)无结构相变,氧化物离子电导率高(约10-2 S/cm)。在工作温度下,对氧的电化学稳定窗口至少为1.2 V。工作环境中的化学稳定性,包括在制备和操作过程中电极/电解质和反应物/电解质界面的反应。只有将电解质导带的底部置于阴极最高已占据分子轨道(HOMO)之上,将电解质价带的顶部置于阳极最低未占据分子轨道(LUMO)之下,才能实现热力学稳定性(图1b)。(11)在整个氧分压和温度范围内,电子导电性(由于电子和空穴)可忽略不计。能够形成致密、气密、无孔的薄膜(约10-20 μm),与正极材料具有良好的附着力,热膨胀系数匹配。电解质和电极(阴极和阳极)之间的界面和电荷转移电阻低(0.1 Ω·cm2),过电位低(图1c)。低成本,无毒,易于制备,在环境条件下化学稳定,特别是在有水分和大气CO2存在的情况下。图2。(a)萤石型(CeO2/ y掺杂ZrO2)(氧化物离子)的理想晶体结构(b)焦绿盐(Gd2Ti2O7)(氧化物离子);(c)钙钛矿型(LaGaO3)(氧化物、质子、电子);(d)褐磨矿(Ba2In2O5)(氧化物离子);(e)有序双钙钛矿(Ba3CaNb2O9)(质子、电子)和(f)层状钙钛矿(Sr2TiO4)(氧化物离子、电子)。单元胞轴方向只显示在非立方结构中。图3。典型固态电解质中离子传导途径(空位和间隙迁移)的示意图。图a和图b分别显示了离子迁移前后离子和空位的位置。虚线圈表示离子迁移后的空缺位置。填充的蓝色圆圈代表掺杂剂。填充的黄色和红色圆圈代表可移动的离子。总离子电导率(体积+晶界)约为10-3 S/cm,在高和低锂活度下的电子电导率可以忽略不计。(28)抗与金属或合金阳极和高压阴极发生化学反应的稳定性。能够使用当前的陶瓷加工技术(如带铸造和薄膜沉积方法)制备致密薄膜(<20 μm)。元素阳极优异的润湿性,具有低离子电荷转移阻力。电化学电位稳定窗口宽,制备方法经济、环保。能够使用高容量的转换电极,如硫和氧。在较高的临界电流密度下不会形成枝晶,并且与电极材料的化学机械性能相匹配。图4。锂和钠离子传导的理想晶体结构。(a) Li4SiO4(锂离子);(b) Li6PS5Cl(锂离子);(c)石榴石型Li7La3Zr2O12(锂离子);(d) Na5SmSi4O12(钠离子);(e) Na3Zr2Si2PO12(单斜钠离子);(f) Na3Zr2Si2PO12(菱形体;Na离子);(g) Na3PS4(钠离子),(h)钙钛矿型La0.67-xLi3x□0.33-2xTiO3(□= a位空位)(锂离子)。单元胞轴方向只显示在非立方结构中。图5。传统锂离子电池、锂金属液体电解质、锂金属固态电解质电池和无阳极固态电池的估计单电池级能量密度比较(采用参考文献(21))。v.t.在此衷心感谢他的博士导师,教授。Jagannatha Gopalakrishnan(已故)和Ashok Kumar Shukla,印度班加罗尔印度科学研究所固态和结构化学部门。谢谢教授们。Werner Weppner和Robert (Bob) Huggins在德国基尔大学学习PDF期间给予他的指导和鼓励。V.T.感谢他的家人和朋友,他所有的学生,以及基尔大学、卡尔加里大学和圣安德鲁斯大学的同事们的支持。本文引用了41个其他出版物。本文档已更新,点击查看更多信息。 这篇文章尚未被其他出版物引用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Current Trends in Solid-State Electrochemical Energy Conversion and Storage Devices
Published as part of ACS Energy Letters special issue “The Evolving Landscape of Energy Research: Views from the Editorial Team”. Figure 1. (a) Schematic representation showing the operating principle of a solid oxide fuel cell (O-SOFC) and ceramic proton conducting H-SOFC. Unlike SOFC, there is no fuel dilution in proton conducting fuel cells since water is produced at the oxygen electrode (cathode). (b) Schematic representation showing a thermodynamically stable cell with proposed/desired energy levels of oxidant and reductant in an electrochemical cell. (c) Schematic representation showing typical polarization curves of “ideal”, high, moderate, and poor ceramic fuel cell performance. High oxide ion conductivity (about 10–2 S/cm) in the intermediate temperature regime (500–750 °C) without structural phase transition. Electrochemical stability window of at least 1.2 V against oxygen at operating temperature. Chemical stability in the working environment including reactions at electrode/electrolyte and reactant/electrolyte interfaces during the preparation and operation. Thermodynamic stability can be achieved only by placing the bottom of the electrolyte conduction band above the highest occupied molecular orbital (HOMO) of the cathode and the top of the electrolyte valence band below the lowest unoccupied molecular orbital (LUMO) of the anode (Figure 1b). (11) Negligible electronic conductivity (due to electrons and holes) over the entire range of oxygen partial pressures and temperatures. Capable of forming dense, gastight, pore-free thin-films (about 10–20 μm) with good adhesion to anode and cathode materials, with matching thermal expansion coefficients. Low interfacial and charge transfer resistances (0.1 Ω·cm2) between the electrolyte and electrodes (cathode and anode) and low ohmic overpotential (Figure 1c). Low-cost, nontoxic, easily prepared, and chemically stable under ambient conditions, particularly in the presence of moisture and atmospheric CO2. Figure 2. Idealized crystal structures of (a) fluorite-type (CeO2/Y-doped ZrO2) (oxide ion); (b) pyrochlore (Gd2Ti2O7) (oxide ion); (c) perovskite-type (LaGaO3) (oxide, proton, electrons); (d) brownmillerite (Ba2In2O5) (oxide ion); (e) ordered double perovskite (Ba3CaNb2O9) (proton, electrons), and (f) layered perovskite (Sr2TiO4) (oxide ion, electrons). Unit cell axis orientation is shown for noncubic structures only. Figure 3. Schematic diagram showing ion conduction pathways (vacancy and interstitial migrations) in typical solid-state electrolytes. Panels a and b show the position of ions and vacancies before and after ion migration, respectively. The dashed circle represents a vacant position after ion migration. Filled blue circles represent dopants. Filled yellow and red circles represent mobile ions. Total (bulk + grain-boundary) ionic conductivity on the order of 10–3 S/cm with negligible electronic conductivity at high and low lithium activities. (28) Stability against chemical reaction with metallic or alloy anodes and high voltage cathodes. Ability to prepare dense thin films (<20 μm) using current ceramic processing technologies such as tape casting and thin-film deposition methods. Excellent wettability of elemental anodes with low ion charge transfer resistance. Wide electrochemical potential stability window, cost-effective method of preparation, and environmentally benign. Ability to work with high-capacity conversion electrodes such as sulfur and oxygen. Free from dendrite formation at higher critical current densities and matching chemomechanical properties with electrode materials. Figure 4. Idealized crystal structures for lithium and sodium ion conduction. (a) Li4SiO4 (lithium ion); (b) Li6PS5Cl (lithium ion); (c) garnet-type Li7La3Zr2O12 (lithium ion); (d) Na5SmSi4O12 (sodium ion); (e) Na3Zr2Si2PO12 (monoclinic; sodium ion); (f) Na3Zr2Si2PO12 (rhombohedral; Na ion); (g) Na3PS4 (sodium ion), and (h) perovskite-type La0.67–xLi3x□0.33–2xTiO3 (□ = A-site vacancy) (lithium ion). Unit cell axis orientation is shown for noncubic structures only. Figure 5. Estimated single cell level energy density comparison for conventional Li ion batteries, Li metal liquid electrolyte, Li metal solid-state electrolyte batteries, and anode-free solid-state batteries (adopted from ref (21)). V. T. would like to sincerely thank his Ph.D. supervisors, Profs. Jagannatha Gopalakrishnan (late) and Ashok Kumar Shukla, at Solid-state and Structural Chemistry Unit, Indian Institute of Science, Bangalore, India. V. T. thanks Profs. Werner Weppner and Robert (Bob) Huggins for their mentorship and encouragement during his PDF at the University of Kiel, Germany. V.T. thanks his family and friends, all his students, and colleagues at University of Kiel, University of Calgary, and University of St. Andrews for their support. This article references 41 other publications. This document has been updated Click for further information. This article has not yet been cited by other publications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


