具有可调机械和粘接性能的自交联嘧啶硅氧烷
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
硅橡胶具有耐高温、耐低温、电绝缘、生物相容性等优异性能。然而,它们的机械强度低是一个致命的缺陷。有必要开发新的分子结构和网络结构,以增强分子间的相互作用,优化有机硅弹性体的性能。因此,本研究提出了一种基于“端功能化物理交联域+长共价主链”网络结构的设计策略。我们开发了具有嵌入光聚合位点的氨基功能化聚硅氧烷(PDMS-NH2)。PDMS-NH2具有较长的聚合物链,以提供共价交联的主链。PDMS-NH2的端基被2-脲基-4[1H]-嘧啶酮(UPy)修饰。改性后的PDMS可以通过端基的物理键域自交联形成超分子弹性体(UPy-PDMS)。同时,设计合成了具有物理键域的热固性有机硅弹性体(r-UPy-PDMS)。UPy-PDMS的存储模量在低频时提高了4个数量级,显著增强了分子间的相互作用。UPy-PDMS硅氧烷含量大于96%。因此,它具有优异的热稳定性(T5% = 447°C, Tmax = 609°C)和耐低温性。UPy-PDMS具有可逆的粘附-拆卸性能。r-UPy-PDMS具有可调的力学性能,抗拉强度范围为0.41 ~ 0.87 MPa。与传统硅橡胶(r-PDMS)相比,这代表了443%的显著改进。本研究旨在设计特定的端基结构和功能,以研究它们对有机硅弹性体机械性能和紧急功能的调节。目的是为加快高强度硅橡胶的发展提供理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
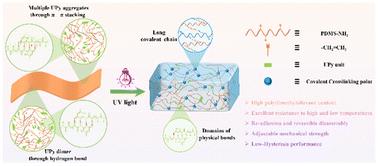
Self-crosslinking pyrimidine siloxanes with tunable mechanical and adhesive properties
Silicone rubbers exhibit high and low temperature resistance, electrical insulation, biocompatibility and other superior properties. However, their low mechanical strength is a fatal defect. It is necessary to develop new molecular structures and network architectures that enhance intermolecular interactions and optimize the performance of silicone elastomers. Consequently, this study presents a design strategy based on an “end-functionalized physical crosslinking domain + long covalent main chain” network structure. We develop amino-functionalized polysiloxane (PDMS-NH2) with embedded photopolymerization sites. PDMS-NH2 has long polymer chains to provide covalent cross-linking of the main chain. The end-groups of PDMS-NH2 are modified by 2-ureido-4[1H]-pyrimidinone (UPy). The modified PDMS can self-crosslink to form supramolecular elastomers (UPy-PDMS) by domains of physical bonds of end groups. Meanwhile, thermoset silicone elastomers enhanced with domains of physical bonds are designed and synthesized (r-UPy-PDMS). The storage modulus of UPy-PDMS increases by four orders of magnitude at low frequencies to significantly enhance intermolecular interactions. UPy-PDMS has a siloxane content greater than 96%. Thereby, it exhibits excellent thermal stability (T5% = 447 °C, Tmax = 609 °C) and low temperature resistance. UPy-PDMS demonstrates reversible adhesion–disassembly performance. r-UPy-PDMS exhibits tunable mechanical properties, with tensile strength ranging from 0.41 to 0.87 MPa. This represents a remarkable 443% improvement compared to conventional silicone rubber (r-PDMS). This study aims to design specific end-group architecture and functionality to investigate their modulation of the mechanical properties and emergent functions of silicone elastomers. The goal is to accelerate the development of high-strength silicone rubbers and provide a theoretical foundation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


