界面Mo-O-W键提高了Bi2MoO6/WO3 Z-Scheme异质结的光降解性能
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
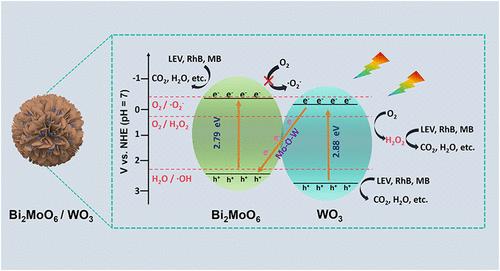
界面工程已被证明是一种很有前途的调节载体传输的策略。本文在Bi2MoO6纳米球和WO3量子点之间构建了一个界面Mo-O-W键。它作为界面光生载流子的快速传递通道,导致直接z型异质结的形成。z型异质结不仅能最大限度地提高氧化还原能力,还能提高光生载流子的分离和转移效率。因此,在LED照射下,它对不同的模拟污染物(左氧氟沙星、罗丹明B和亚甲基蓝)表现出优异的降解活性和可重复使用性,其光催化速率约为两倍。出乎意料的是,通过直接一步双电子氧还原反应过程形成了相当数量的H2O2,在光降解过程中发挥了重要作用,甚至超过了电子/空穴作为活性物质的作用。进一步分析了左氧氟沙星在z型异质结下的光降解途径。该研究为建立界面化学键、调节电荷转移和光降解机制提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interfacial Mo–O–W Bonds Improve the Photodegradation Performance of a Bi2MoO6/WO3 Z-Scheme Heterojunction
Interface engineering has been proven to be a promising strategy for regulating carrier transport. Herein, an interfacial Mo–O–W bond was constructed between Bi2MoO6 nanospheres and WO3 quantum dots. It served as a fast transfer channel for interfacial photogenerated carriers, resulting in the formation of a direct Z-scheme heterojunction. The Z-scheme heterojunction not only maximized the redox ability but also exhibited enhanced separation and transfer efficiency of photogenerated carriers. Thereby, it exhibited excellent degradation activity and reusability for different simulated pollutants (levofloxacin, rhodamine B, and methylene blue) under LED irradiation, and its photocatalytic rate is approximately doubled. Unexpectedly, a considerable amount of H2O2 was formed via a direct one-step two-electron oxygen reduction reaction process, which played a significant role in the photodegradation process, even surpassing the role of electrons/holes as active species. Furthermore, the photodegradation pathway of levofloxacin by the Z-scheme heterojunction was analyzed. This study could provide insights into establishing an interfacial chemical bond, modulating charge transfer, and the photodegradation mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


