4HPR纳米制剂调节MAPKAPK3/3pK信号,控制Bax磷酸化和线粒体易位,实现神经母细胞瘤细胞凋亡。
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Nanomedicine : nanotechnology, biology, and medicine
Pub Date : 2025-09-19
DOI:10.1016/j.nano.2025.102858
引用次数: 0
摘要
神经母细胞瘤(Neuroblastoma, NB)是一种源自神经嵴祖细胞的神经内分泌肿瘤,常见于交感神经系统,尤其是肾上腺髓质。尽管治疗进展,晚期NB的预后仍然很差,需要改进治疗方案。4HPR对包括NB在内的多种肿瘤均有细胞毒性,但具有较低的全身毒性;然而,其临床应用受到溶解度和生物利用度差的限制。为了解决这个问题,我们开发了一种基于人血清白蛋白的4HPR纳米制剂,使用简单的溶解方法。该制剂有效诱导NB细胞凋亡,表现为ROS生成增加,Bax/Bcl-2比值升高,细胞脱离增强。值得注意的是,我们首次发现MAPKAPK3下调导致Bax磷酸化减少和线粒体易位增加。共免疫沉淀证实了MAPKAPK3-Bax的直接相互作用,表明MAPKAPK3通过磷酸化调节Bax。我们的纳米制剂可以调节这种串扰,作为神经母细胞瘤的一种新的治疗策略,显示出有希望的转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
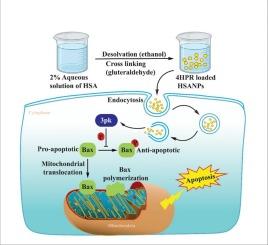
4HPR nanoformulation regulates MAPKAPK3/3pK signaling to control Bax phosphorylation and mitochondrial translocation to execute apoptosis in neuroblastoma
Neuroblastoma (NB) is a neuroendocrine tumor derived from neural crest progenitor cells, commonly arising along the sympathetic nervous system, especially in the adrenal medulla. Despite therapeutic advances, the prognosis for advanced-stage NB remains poor, necessitating improved treatment options. 4HPR has demonstrated cytotoxicity in various tumors, including NB, with low systemic toxicity; however, its clinical use is restricted by poor solubility and bioavailability. To address this, we developed a human serum albumin-based nanoformulation of 4HPR using a simple desolvation method. This formulation effectively induced apoptosis in NB cells, marked by increased ROS generation, elevated Bax/Bcl-2 ratio, and enhanced cell detachment. Notably, we identified for the first time that MAPKAPK3 downregulation leads to reduced Bax phosphorylation and increased mitochondrial translocation. Co-immunoprecipitation confirmed a direct MAPKAPK3–Bax interaction, indicating MAPKAPK3 regulates Bax via phosphorylation. Our nanoformulation modulates this cross-talk, demonstrating promising translational potential as a novel therapeutic strategy for neuroblastoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
0.00%
发文量
133
审稿时长
42 days
期刊介绍:
The mission of Nanomedicine: Nanotechnology, Biology, and Medicine (Nanomedicine: NBM) is to promote the emerging interdisciplinary field of nanomedicine.
Nanomedicine: NBM is an international, peer-reviewed journal presenting novel, significant, and interdisciplinary theoretical and experimental results related to nanoscience and nanotechnology in the life and health sciences. Content includes basic, translational, and clinical research addressing diagnosis, treatment, monitoring, prediction, and prevention of diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


