继发于人类ILK错义变异的mTOR信号增加抑制代谢降低的肾发生。
IF 5.1
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
肾脏形成对哺乳动物一生的肾脏功能至关重要。肾元形成减少是一个依赖于输尿管-间质组织相互作用的胚胎过程,是先天性肾尿路异常(先天性肾尿路异常)的基本特征,也是成人发病的心血管和肾脏疾病的前兆。然而,控制胚胎发生过程中肾单位数量的机制在很大程度上是不明确的。在这里,我们阐明了mTOR信号增加对小鼠肾形成的有害影响。在人类CAKUT队列中发现了一种罕见的人类整合素连接激酶(ILK)遗传错义变异(ILKT173I)。用IlkT173I替代小鼠ILKWT等位基因导致肾脏mTOR信号增加,肾细胞数量减少,输尿管分支减少,后者可通过雷帕霉素恢复。胚胎肾细胞的转录组学分析表明,mTOR信号的升高仅限于非输尿管间质,原位免疫染色证实了这一发现。肾源特异性标志物、形态学分析和肾源性祖细胞的增殖表明,ilkt173i敲入肾脏的肾源性细胞成熟减少。非输尿管细胞的代谢谱显示氧化ATP产生减少。总之,我们的数据揭示了ILKT173I下游过量mTOR信号通过抑制肾源性细胞谱系中的成熟、细胞增殖和代谢而起的有害作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
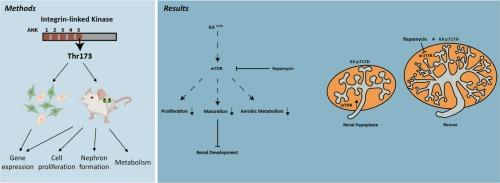
Increased mTOR signaling secondary to a human ILK missense variant inhibits nephrogenesis with decreased metabolism
Nephrogenesis is critical to mammalian kidney function throughout life. Decreased nephron formation, an embryonic process dependent on ureteric-mesenchymal tissue interactions, is a fundamental feature of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) and an antecedent to adult-onset cardiovascular and renal disease. Yet, mechanisms controlling the number of nephrons formed during embryogenesis are largely undefined. Here, we elucidated deleterious effects of increased mTOR signaling on murine nephrogenesis. A rare human genetic missense variant (ILKT173I) in Integrin-Linked Kinase (ILK) was identified in a human CAKUT cohort. Replacement of the mouse ILKWT allele with IlkT173I caused increased kidney mTOR signaling, low nephron number, and decreased ureteric branching, the latter of which was rescued by rapamycin. Transcriptomic analysis of sorted embryonic kidney cells suggested that elevated mTOR signaling is limited to non-ureteric mesenchyme, a finding that was substantiated by immunostaining in situ. Maturation of nephrogenic cells in IlkT173I-knock-in kidneys was decreased as demonstrated by nephrogenic-specific markers, morphologic analysis, and proliferation of nephrogenic progenitors. Metabolic profiling of non-ureteric cells demonstrated decreased oxidative ATP production. Together, our data revealed a deleterious role of excessive mTOR signaling downstream of ILKT173I by inhibiting maturation, cell proliferation and metabolism in the nephrogenic cell lineage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
1.90%
发文量
79
审稿时长
32 days
期刊介绍:
Mechanisms of Ageing and Development is a multidisciplinary journal aimed at revealing the molecular, biochemical and biological mechanisms that underlie the processes of aging and development in various species as well as of age-associated diseases. Emphasis is placed on investigations that delineate the contribution of macromolecular damage and cytotoxicity, genetic programs, epigenetics and genetic instability, mitochondrial function, alterations of metabolism and innovative anti-aging approaches. For all of the mentioned studies it is necessary to address the underlying mechanisms.
Mechanisms of Ageing and Development publishes original research, review and mini-review articles. The journal also publishes Special Issues that focus on emerging research areas. Special issues may include all types of articles following peered review. Proposals should be sent directly to the Editor-in-Chief.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


