胰岛素受体pY1150的体内靶向:调节结直肠癌细胞信号通路的新途径。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
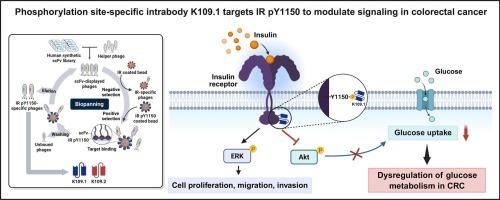
胰岛素受体(IR)的激活对生理代谢、生长和增殖的调节至关重要,并与包括结直肠癌(CRC)在内的多种癌症有关。在IR细胞内激酶结构域的酪氨酸残基中,Tyr1150通过调节底物与激酶活性位点的结合,在受体激活中起关键作用。在这项研究中,我们介绍了一种新的方法,通过开发体内靶向磷酸化Tyr1150 (IR pY1150)来选择性调节CRC中的胰岛素信号。利用噬菌体展示技术,我们从人单链可变片段(scFv)抗体库中分离出磷酸化位点特异性单链可变片段K109.1,该片段特异性结合IR pY1150。K109.1随后被设计为在细胞内发挥作用的体内药物。结直肠癌细胞中K109.1的异位表达选择性地抑制胰岛素介导的关键下游效应物的磷酸化,包括胰岛素受体底物和Akt,从而导致胰岛素依赖性葡萄糖摄取显著减少。值得注意的是,K109.1不影响细胞外信号调节的激酶磷酸化或改变细胞增殖、迁移或侵袭。我们进一步在BT-474和HEK293细胞中评估K109.1,以评估其在其他细胞模型中的作用。在BT-474乳腺癌细胞中,K109.1选择性地抑制Akt磷酸化,而在HEK293细胞中,K109.1同时抑制Akt和ERK磷酸化,表明了上下文特异性的信号反应。综上所述,这些发现表明,体内介导的IR pY1150靶向对于调节葡萄糖代谢至关重要,这表明所开发的抗体K109.1可以作为调节胰岛素介导的信号通路的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Intrabody targeting of insulin receptor pY1150: A novel approach to modulate signaling pathways in colorectal cancer cells
The activation of the insulin receptor (IR) is central to the regulation of physiological metabolism, growth, and proliferation, and is associated with various cancers, including colorectal cancer (CRC). Among the tyrosine residues in the intracellular kinase domain of IR, Tyr1150 plays a pivotal role in receptor activation by regulating substrate binding to the kinase active site. In this study, we introduce a novel approach for selectively modulating insulin signaling in CRC through the development of an intrabody targeting phosphorylated Tyr1150 (IR pY1150). Using phage display technology, we isolated a phosphorylation site-specific single-chain variable fragment (scFv), K109.1, from a human scFv antibody library, which specifically binds to IR pY1150. K109.1 was subsequently engineered as an intrabody designed to function within cells. Ectopic expression of K109.1 in CRC cells selectively inhibited insulin-mediated phosphorylation of key downstream effectors, including insulin receptor substrates and Akt, thereby leading to a significant reduction in insulin-dependent glucose uptake. Notably, K109.1 did not affect extracellular signal-regulated kinase phosphorylation or alter cell proliferation, migration, or invasion. We further evaluated K109.1 in BT-474 and HEK293 cells to assess its effects in additional cellular models. In BT-474 breast cancer cells, K109.1 selectively inhibited Akt phosphorylation, while in HEK293 cells it suppressed both Akt and ERK phosphorylation, indicating context-specific signaling responses. Taken together, these findings indicate that intrabody-mediated targeting of IR pY1150 is crucial for regulating glucose metabolism, suggesting that the developed antibody, K109.1, could serve as a tool for modulating insulin-mediated signaling pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


