宿主-微生物-寄生虫串扰:肠道菌群失调通过改变免疫和甘油磷脂代谢加剧了婴儿利什曼原虫的发病机制。
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
摘要
内脏利什曼病是一种致命的寄生虫病,如果不治疗,迫切需要新的治疗策略。肠道微生物群可以调节宿主免疫和疾病易感性,但其对内脏利什曼病的具体影响及其潜在的分子机制尚不清楚。本研究在小鼠模型中研究了肠道微生物群与婴儿利什曼原虫感染之间的相互作用,重点关注寄生虫载量、免疫反应和代谢变化。诱导肠道失调小鼠感染婴儿乳杆菌并监测5周。安乐死后,评估小鼠肝脏和脾脏的感染状况,分析血浆中的抗体、细胞因子和LC-MS/ ms - s代谢组学,对肝脏组织进行转录组学测序,并对粪便样本进行16S rRNA测序。结果表明,乳杆菌感染破坏了肠道菌群的多样性,减少了Clostridia_UCG-014和Lachnospiraceae_NK4A136_group等类群,引起了Muribaculaceae减少和Bacteroides增加等生态失调相关的变化(p值为0.0018 ~ 0.0472)。生态失调小鼠的肝脏和脾脏的寄生虫负荷较高,炎症病变较多,血清IgG水平较低,Th1-和th2型细胞因子升高。转录组学和代谢组学分析显示脂质代谢失调(如胆固醇和脂肪酸生物合成)和甘油磷脂增加,可能支持寄生虫膜合成。这些发现表明,肠道菌群失调通过改变宿主免疫和代谢途径加剧了婴儿乳杆菌的发病机制,突出了针对微生物群的干预措施对抗寄生虫感染的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
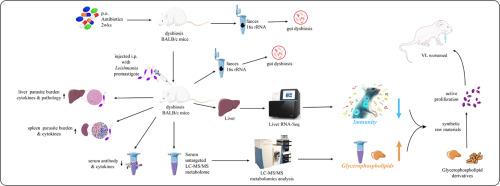
Host-microbiota-parasite crosstalk: Gut microbiota dysbiosis exacerbates Leishmania infantum pathogenesis through altered immunity and glycerylphosphatide metabolism
Visceral leishmaniasis, a deadly parasitic disease if untreated, urgently requires novel therapeutic strategies. The gut microbiota can modulate host immunity and disease susceptibility, but its specific impact on visceral leishmaniasis and the underlying molecular mechanisms are unclear. This study examined the interaction between intestinal microbiota and Leishmania infantum infection in a murine model, focusing on parasite load, immune response, and metabolic changes. Mice with induced intestinal dysbiosis were infected with L. infantum and monitored for five weeks. Post-euthanasia, murine liver and spleen were assessed for infection status, plasma was analyzed for antibodies, cytokines, and LC-MS/MS-based metabolomics, liver tissues were sequenced for transcriptomics, and fecal samples were analyzed by 16S rRNA sequencing. Results showed that L. infantum infection disrupted gut microbiota diversity, reducing taxa such as Clostridia_UCG-014 and Lachnospiraceae_NK4A136_group, and inducing dysbiosis-related changes such as decreased Muribaculaceae and increased Bacteroides (P-values from 0.0018 to 0.0472). Dysbiotic mice had higher parasite loads in liver and spleen, more inflammatory lesions, lower serum IgG levels, and elevated Th1- and Th2-type cytokines. Transcriptomic and metabolomic analyses revealed dysregulated lipid metabolism (e.g., cholesterol and fatty acid biosynthesis) and increased glycerophospholipids, potentially supporting parasite membrane synthesis. These findings demonstrate that gut microbiota dysbiosis exacerbates L. infantum pathogenesis by altering host immunity and metabolic pathways, highlighting the potential for microbiota-targeted interventions to combat parasitic infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


