长双歧杆菌的胞外多糖。婴儿和青少年双歧杆菌保护肠道上皮细胞免受抗生素诱导的破坏,但不影响上皮细胞对大肠杆菌的反应
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
抗生素的使用是抗菌素耐药性(AMR)的主要原因,并可能损害肠道上皮的完整性。然而,减轻这些副作用的策略仍然有限。本研究研究了来自婴儿双歧杆菌和青少年双歧杆菌的外多糖(EPS)是否能保护肠上皮细胞免受多西环素(DOX)和氧化锌(ZnO)造成的屏障破坏。在T84细胞中,我们发现DOX和ZnO均显著降低了经上皮电阻(TEER),表明屏障功能受损。来自青春期白僵菌的EPS有效地保持了屏障的完整性。相比之下,来自婴儿B.的EPS仅对较高浓度的DOX具有保护作用。DOX和ZnO下调了紧密连接和炎症通路相关的基因。EPS对基因表达的影响是菌株特异性的,部分是恢复性的。IL-8分泌增加,并在DOX暴露期间受到调节,提示免疫通路参与。相比之下,EPS类型均不影响Caco-2细胞中的大肠杆菌O119粘附或炎症反应。这些发现表明,双歧杆菌EPS可以以结构和菌株依赖的方式减轻抗生素诱导的上皮损伤,支持它们作为抗菌治疗期间保护肠道屏障功能的辅助剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
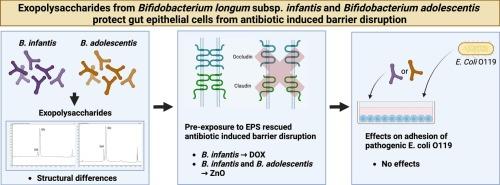
Exopolysaccharides from Bifidobacterium longum supsp. Infantis and Bifidobacterium adolescentis protect gut epithelial cells from antibiotic- induced disruption but do not affect epithelial responses to Escherichia Coli
Antibiotic use is a major contributor to antimicrobial resistance (AMR) and can compromise gut epithelial integrity. However, strategies to mitigate these side effects remain limited. This study investigated whether exopolysaccharides (EPS) derived from Bifidobacterium infantis and Bifidobacterium adolescentis can protect intestinal epithelial cells from barrier disruption caused by doxycycline (DOX) and zinc oxide (ZnO). Using T84 cells, we found that both DOX and ZnO significantly reduced transepithelial electrical resistance (TEER), indicating impaired barrier function. EPS from B. adolescentis effectively preserved barrier integrity against both agents. In contrast, EPS from B. infantis was protective only against DOX at higher concentrations. DOX and ZnO downregulated genes involved in tight junction and inflammatory pathways. EPS effects on gene expression were strain-specific and partly restorative. IL-8 secretion was enhanced by B. adolescentis EPS and modulated during DOX exposure, suggesting immune pathway involvement. In contrast, neither EPS type affected Escherichia coli O119 adhesion or inflammatory responses in Caco-2 cells. These findings demonstrate that bifidobacterial EPS can mitigate antibiotic-induced epithelial damage in a structure- and strain-dependent manner, supporting their potential as adjunctive agents to preserve gut barrier function during antimicrobial treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


