ZnO/MgO和Zn/ mg改性多孔沸石作为核心-卫星材料调节水蒸气吸附性能的协同效应
IF 8.7
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
湿度控制是工业过程中的一个主要问题,本文主要研究了核心卫星材料:Zeo-X-Mg、Zeo-X-Zn、Zeo-X-ZnO和Zeo-X-MgO的合成,并对它们的水蒸气吸附性能进行了比较研究。采用Langmuir、Freundlich、Sips和Guggenheim-Anderson-De Boer (GAB)等温模型分析了吸附机理。采用非原位离子交换辅助水热法合成所得材料,并采用x射线衍射(XRD)、x射线荧光(XRF)、固态魔角旋转(MAS)-核磁共振(NMR)、场发射扫描电镜(FE-SEM)/能量色散x射线能谱(EDX)、热重分析(TGA)-差示扫描量热法(DSC)、N₂吸附和傅里叶变换红外光谱(FTIR)技术对材料进行了表征。XRD分析表明,由于ZnO、Zn(OH) 2、MgO和Mg(OH) 2在Zeo-X-Na表面的存在,Zeo-X-ZnO和Zeo-X-MgO样品中存在额外的相。SEM/EDX分析显示,与钠石笼的六元环(D6R)相对应的八面体颗粒均匀,Mg和Zn元素分布均匀。Zeo-X-ZnO、Zeo-X-MgO、Zeo-X-Zn、Zeo-X-Mg和Zeo-X-Na样品的水蒸气吸附量分别为26.9、25.1、21.5、18.1和14.1 mmol/g。尽管孔隙率低,但Zeo-X-ZnO和Zeo-X-MgO的吸附能力高于用Mg 2 +、Zn 2 +和纯沸石交换的样品。对于Zeo-X-MgO和Zeo-X-ZnO样品,由于在水化过程中形成Zn(OH) 2和Mg(OH) 2配合物,水分子的吸附优先发生在沸石结构表面。GAB模型显示出较高的R²值和较低的误差函数值,表明在变压力下在无限单层和多层上的吸附。Freundlich模型表明,吸附过程中在吸附剂表面发生了化学吸附。Zeo-X-Na、Zeo-X-Zn、Zeo-X-ZnO、Zeo-X-Mg和Zeo-X-MgO的等等吸附热分别为53.22、63.03、80.96、65.23和73.15 kJ/mol。这些结果指出了一种涉及水蒸气的工业过程的创新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
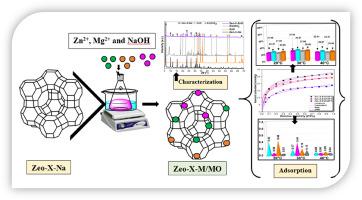
Insights into the synergistic effects of ZnO/MgO and Zn/Mg-modified porous zeolite as core-satellite materials for tuning water vapor sorption properties
Humidity control is a major issue in industrial processes, the present work focuses on the synthesis of the core-satellite materials: Zeo-X-Mg, Zeo-X-Zn, Zeo-X-ZnO and Zeo-X-MgO, followed by a comparative study of their water vapor adsorption properties. The adsorption mechanism was elucidated using isothermal modeling with Langmuir, Freundlich, Sips and Guggenheim-Anderson-De Boer (GAB) models. The obtained materials were synthesized by an ex situ ion-exchange assisted hydrothermal method and characterized using X-ray diffraction (XRD), X-ray fluorescence (XRF), solid-state magic angle spinning (MAS)-nuclear magnetic resonance (NMR), field emission scanning electron microscopy (FE-SEM)/energy dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA)-differential scanning calorimetry (DSC), N₂ sorption and Fourier transform infrared spectroscopy (FTIR) techniques. XRD analysis revealed additional phases in the Zeo-X-ZnO and Zeo-X-MgO samples due to the presence of ZnO, Zn(OH)₂, MgO, and Mg(OH)₂ on the Zeo-X-Na sample's surface. SEM/EDX analysis revealed uniform octahedral particles corresponding to the six-membered rings (D6R) of sodalite cages and a homogeneous distribution of Mg and Zn elements. The water vapor adsorption capacities were 26.9, 25.1, 21.5, 18.1, and 14.1 mmol/g for Zeo-X-ZnO, Zeo-X-MgO, Zeo-X-Zn, Zeo-X-Mg, and Zeo-X-Na samples, respectively. Despite their low porosities, Zeo-X-ZnO and Zeo-X-MgO showed higher adsorption capacities comparable to samples exchanged with Mg²⁺, Zn²⁺ and pure zeolite. For Zeo-X-MgO and Zeo-X-ZnO samples, water molecule adsorption occurs preferentially on the surface of the zeolite structure, due to the formation of Zn(OH)₂ and Mg(OH)₂ complexes during to the hydration. The GAB model showed a higher R² value with low values of the error functions, indicating adsorption on infinite monolayers and multilayers at variable pressure. The Freundlich model showed that chemisorption occurred at the adsorbent surface during adsorption. The obtained isosteric heat of adsorption were 53.22, 63.03, 80.96, 65.23, and 73.15 kJ/mol for Zeo-X-Na, Zeo-X-Zn, Zeo-X-ZnO, Zeo-X-Mg, and Zeo-X-MgO, respectively. These results point to an innovative approach to industrial processes involving water vapor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


