Nb5+掺杂可调节尖晶石LiMn2O4的晶体形态和电子结构
IF 4.3
2区 材料科学
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
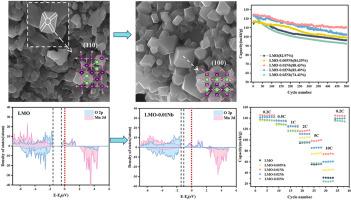
锰溶解和Jahn-Teller畸变是限制尖晶石LiMn2O4循环寿命的主要因素。本研究通过简单的高温固相法成功合成了Nb5+掺杂的截断八面体Li1.05Mn2-xNbxO4(0≤x≤0.03)。Nb5+的掺杂不仅增强了LiMn2O4的结构稳定性,而且提高了Li +的扩散速率。SEM分析表明,Nb5+的掺杂有效抑制了(110)平面的生长,从而减轻了Mn的溶解。同时,TEM结果表明,掺杂后形成了更薄的阴极电解质界面膜,有利于提高循环稳定性。进一步的DFT计算证实,Nb5+掺杂通过双重机制提高了LiMn2O4的结构稳定性:降低了Mn- eg轨道的占位,增强了Mn- o键能。另一方面,Nb5+的掺杂扩展了晶格,CV和EIS测试显示Li+的扩散速率增加。PDOS计算显示,带隙变窄,提高了电子导电性,从而赋予LiMn2O4高速率性能。因此,Li1.05Mn2-xNbxO4具有优异的倍率性能和延长的循环寿命。具体而言,li1.05 mn1.99 nb0.0104在1 C下循环500次后的初始放电容量为124.61 mAh/g,容量保留率为88.43%,即使在10 C下也保持74.61 mAh/g。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nb5+ doping modulates crystal morphology and electronic structure of spinel LiMn2O4 for high-rate long-cycle cathode materials
Manganese dissolution and the Jahn-Teller distortion represent the primary factors limiting the cycle life of spinel LiMn2O4. In this study, Nb5+-doped Li1.05Mn2-xNbxO4 (0 ≤ x ≤ 0.03) with truncated octahedral morphology was successfully synthesized via a simple high-temperature solid-state method. Nb5+ doping not only enhanced the structural stability of LiMn2O4 but also increased the Li + diffusion rate. SEM analysis revealed that Nb5+ doping effectively suppressed (110) plane growth, thereby mitigating Mn dissolution. Simultaneously, TEM results indicated that a thinner cathode electrolyte interphase film was formed upon doping, which contributed to enhanced cycling stability. Further DFT calculations confirmed that Nb5+ doping improved the structural stability of LiMn2O4 through a dual mechanism: reducing the occupancy of the Mn eg orbitals and strengthening the Mn-O bonding energy. On the other hand, Nb5+ doping expands the lattice, with CV and EIS tests showing increased Li+ diffusion rates. PDOS calculations revealed a narrowed band gap, which improved the electronic conductivity, thereby endowing LiMn2O4 with high-rate performance. Hence, Li1.05Mn2-xNbxO4 exhibits superior rate capability and extended cycle life. Specifically, Li1.05Mn1.99Nb0.01O4 delivered an initial discharge capacity of 124.61 mAh/g with 88.43 % capacity retention after 500 cycles at 1 C, and maintained 74.61 mAh/g even at 10 C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Particuology
工程技术-材料科学:综合
CiteScore
6.70
自引率
2.90%
发文量
1730
审稿时长
32 days
期刊介绍:
The word ‘particuology’ was coined to parallel the discipline for the science and technology of particles.
Particuology is an interdisciplinary journal that publishes frontier research articles and critical reviews on the discovery, formulation and engineering of particulate materials, processes and systems. It especially welcomes contributions utilising advanced theoretical, modelling and measurement methods to enable the discovery and creation of new particulate materials, and the manufacturing of functional particulate-based products, such as sensors.
Papers are handled by Thematic Editors who oversee contributions from specific subject fields. These fields are classified into: Particle Synthesis and Modification; Particle Characterization and Measurement; Granular Systems and Bulk Solids Technology; Fluidization and Particle-Fluid Systems; Aerosols; and Applications of Particle Technology.
Key topics concerning the creation and processing of particulates include:
-Modelling and simulation of particle formation, collective behaviour of particles and systems for particle production over a broad spectrum of length scales
-Mining of experimental data for particle synthesis and surface properties to facilitate the creation of new materials and processes
-Particle design and preparation including controlled response and sensing functionalities in formation, delivery systems and biological systems, etc.
-Experimental and computational methods for visualization and analysis of particulate system.
These topics are broadly relevant to the production of materials, pharmaceuticals and food, and to the conversion of energy resources to fuels and protection of the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


