靶向伴侣介导的自噬调节破骨细胞活性作为骨质疏松症的治疗策略
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
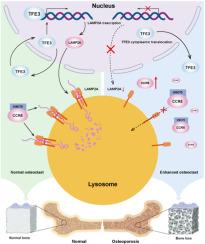
骨质疏松症是一种与年龄相关的骨代谢疾病,其特征是持续的骨量减少和骨结构破坏。破骨细胞是骨重塑过程中的重要细胞,与骨质疏松症的发生和发展密切相关;然而,相关的监管机制仍不清楚。在本研究中,TFE3细胞质易位抑制老年骨质疏松患者破骨细胞和前体中LAMP2A的表达,并且下调LAMP2A表达介导了伴侣介导的自噬(CMA)的衰减。这种抑制抑制了细胞内CCR5的降解,增加了破骨细胞前体细胞的破骨细胞分化,增强了成熟破骨细胞的骨吸收活性,导致骨丢失和重塑。此外,我们构建了携带CMA激活剂的破骨细胞靶向纳米颗粒,并证明在体内增强破骨细胞CMA活性可以抑制破骨细胞异常的骨吸收活性,从而有效增加骨量,缓解骨质疏松症的进展。本研究发现,在体内和体外,破骨细胞及其前体中lamp2a介导的CMA活性负向调节破骨细胞分化和骨吸收活性。lamp2a介导的CMA活性的衰减在骨质疏松症的发生发展中起着重要作用,增强lamp2a介导的CMA活性是骨质疏松症的潜在治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting chaperone-mediated autophagy to regulate osteoclast activity as a therapeutic strategy for osteoporosis
Osteoporosis is an age-related bone metabolic disease characterized by a persistent bone mass decrease and bone structure destruction. Osteoclasts, important cells in the bone remodelling process, are closely associated with the onset and progression of osteoporosis; however, the regulatory mechanisms involved remain unclear. In this study, TFE3 cytoplasmic translocation inhibited LAMP2A expression in osteoclasts and precursors in elderly individuals with osteoporosis and the downregulation of LAMP2A expression mediated the attenuation of chaperone-mediated autophagy (CMA). This inhibition prevented intracellular CCR5 degradation, increased the osteoclast differentiation of osteoclast precursor cells, and enhanced the bone resorption activity of mature osteoclasts, leading to bone loss and remodelling. In addition, we constructed osteoclast-targeted nanoparticles carrying CMA activators and demonstrated that enhancing osteoclast CMA activity in vivo inhibited the abnormal bone resorption activity of osteoclasts, thereby effectively increasing bone mass and alleviating osteoporosis progression. This study revealed that LAMP2A-mediated CMA activity in osteoclasts and their precursors negatively regulates osteoclast differentiation and bone resorption activities both in vivo and in vitro. The attenuation of LAMP2A-mediated CMA activity plays an important role in the development of osteoporosis, and enhancing LAMP2A-mediated CMA activity represents a potential therapeutic strategy for osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


