使用人原代肺细胞研究呼吸道病毒感染的简单和高遏制肺芯片模型
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
冠状病毒和流感等空气传播的呼吸道病毒对公共卫生和经济构成重大威胁,COVID-19大流行凸显了这一点。临床前研究受到一些模型的阻碍,这些模型不能很好地模拟人体组织结构和功能,通常依赖于永生化细胞系和低通量的动物研究。这限制了对疾病机制、药物作用和靶标适宜性的准确预测。在这里,我们报道了一种定制设计的、被动流动的、高密封芯片,用于在大面积膜上的气液界面(ALI)培养人原代支气管上皮细胞(hPBECs)。双室微流控芯片,由水平支撑膜分开,被封闭在一个35毫米密封的培养皿中,可以在标准孵化器中安全使用,没有泄漏或生物安全问题。该平台支持高分辨率原位成像、根尖病毒感染和细胞和分泌物(如粘液、病毒裂解液)的分子分析检索。我们在ALI培养了4周的分化hPBECs中证明了人类冠状病毒NL63 (HCoV-NL63)的强大感染和复制。免疫荧光法证实上皮细胞分化(如纤毛细胞),RT-qPCR法监测感染动力学,持续7天。基于干扰素的免疫反应显示出活性增加,病毒反应途径(如复制、炎症、免疫调节)上调,并且在供体(如ISG15、IFIT1)中一致激活。总的来说,我们提出了一个可重复的,小规模的芯片模型,能够在体外研究呼吸道病毒及其对人气道上皮的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
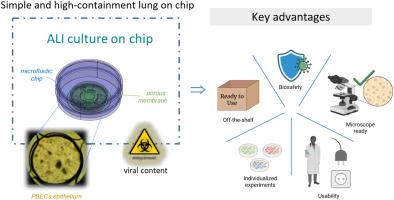
Simple and high-containment lung-on-chip model for studying respiratory viral infections using human primary lung cells
Airborne respiratory viruses, such as coronaviruses and influenza, pose major threats to public health and the economy, as highlighted by the COVID-19 pandemic. Preclinical research is hindered by models that poorly mimic human tissue structure and function, often relying on immortalized cell lines and low-throughput animal studies. This limits accurate prediction of disease mechanisms, drug effects, and target suitability. Here, we report a custom-engineered, passive-flow, high-containment chip for culturing human primary bronchial epithelial cells (hPBECs) at air-liquid interface (ALI) on a large-area membrane. The dual-chamber microfluidic chip, separated by a horizontal support membrane, is enclosed in a 35 mm sealed Petri dish, enabling safe use in standard incubators without leakage or biosafety concerns. The platform supports high-resolution in-situ imaging, apical viral infection, and retrieval of cells and secretions (e.g., mucus, viral lysate) for molecular analysis. We demonstrate robust infection and replication of human coronavirus NL63 (HCoV-NL63) in differentiated hPBECs cultured up to 4 weeks at ALI. Epithelial differentiation was confirmed by immunofluorescence (e.g., ciliated cells), and infection kinetics were monitored by RT-qPCR over 7 days. The interferon-based immune response showed increased activity, with upregulation of viral response pathways (e.g., replication, inflammation, immunoregulation), and consistent activation across donors (e.g., ISG15, IFIT1). Collectively, we present a reproducible, small-scale chip model that enables high-containment in vitro studies of respiratory viruses and their effects on human airway epithelia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


