构建一种高性能镁单原子催化剂用于生物质衍生羰基化合物在乙醇中的转移加氢
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
近年来,由金属-有机配位催化剂和醇类组成的内源氢体系被广泛应用于生物质羰基化合物的转移加氢反应。金属-有机配位催化剂对仲醇的TH反应能力较好,但大多不能有效地利用便宜的伯醇作为供氢体。此外,它们通常含有较高的金属含量,这也在很大程度上导致了催化效率低。本文采用自组装和热解相结合的方法,构建了一种新型镁单原子催化剂(Mg- nc),其Mg质量分数仅为0.37 wt%。表征结果表明,Mg在Mg- nc中原子分散,并与四个吡啶- n配位。由于Mg向配位的吡啶- n有明显的电子转移,Mg - n4活性中心表现出较高的路易斯酸碱强度,且含量丰富,具有显著的催化活性。当Mg-NC用于5-羟甲基糠醛(HMF)在乙醇(EtOH)中的TH时,2,5-双(羟甲基)呋喃(BHMF)的产率高达96.3%,在150°C下反应5 h,产率为19.85 molBHMF molMg−1 h−1。更有趣的是,在EtOH中,Mg-NC上TH的过程被证明是通过氢自由基机制进行的。Mg-NC具有较强的催化通用性;它不仅可以利用其他伯醇(如正丙醇和正丁醇)作为给氢体,还可以催化其他羰基化合物(如糠醛、5-甲基糠醛、苯甲醛、环己酮、乙酰丙酸)的TH。综上所述,本研究为加强各种生物质衍生羰基化合物TH中的伯醇对高价值精细化学品的供氢能力提供了重要线索和参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
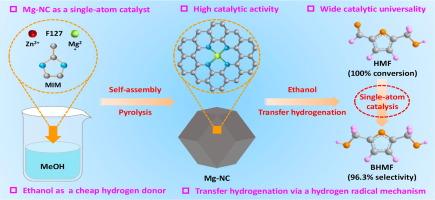
Constructing a high-performance magnesium single-atom catalyst for the transfer hydrogenation of biomass-derived carbonyl compounds in ethanol
Endogenous hydrogen systems, consisting of metal–organic coordination catalysts and alcohols, have been widely applied for the transfer hydrogenation (TH) of biomass-derived carbonyl compounds in recent years. Metal-organic coordination catalysts showed satisfactory ability of TH in the secondary alcohols, but most of them could not effectively employ the cheaper primary alcohols as hydrogen donors. Furthermore, they commonly contained high metal contents, which also led to low catalytic efficiency in significant measure. In this work, we constructed a novel magnesium single-atom catalyst (Mg-NC) with merely 0.37 wt% Mg by means of a combined self-assembly and pyrolysis strategy. The characterization results indicated that Mg was atomically dispersed and it was coordinated with four pyridinic-N in Mg-NC. Due to the obvious electron transfer from Mg to its coordinated pyridinic-N, Mg–N4 active centers displayed high Lewis acid-base strength with abundant content, which brought remarkable catalytic activity. When Mg-NC was used for the TH of 5-hydroxymethylfurfural (HMF) in ethanol (EtOH), 2,5-bis(hydroxymethyl)furan (BHMF) yield was up to 96.3 % with high productivity of 19.85 molBHMF molMg−1 h−1 at 150 °C for 5 h. More interestingly, the process of TH over Mg-NC in EtOH was proved to proceed via the hydrogen radical mechanism. Additionally, Mg-NC exhibited powerful catalytic universality; it could not only utilize other primary alcohols (such as n-propanol and n-butanol) as hydrogen donors, but also catalyze the TH of other carbonyl compounds (such as furfural, 5-methylfurfural, benzaldehyde, cyclohexanone, and levulinic acid). Overall, this work offered some important clues and references to reinforce the hydrogen-supplying ability of primary alcohols in the TH of various biomass-derived carbonyl compounds to high-value fine chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


