生物相容性木质素-麦角硫因复合物的超声纳米工程用于靶向破膜和持续口服根除幽门螺杆菌
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-18
DOI:10.1016/j.jddst.2025.107545
引用次数: 0
摘要
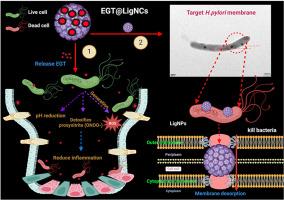
幽门螺杆菌(h.p ylori)是一种革兰氏阴性螺旋形细菌,全球约有44亿人感染。本研究采用高强度超声溶剂处理和均质化相结合的新方法,对木质素纳米颗粒(LigNPs)进行了功能化和纳米转化,将天然疏水抗氧化剂麦角硫因(EGT)接枝到木质素纳米颗粒(EGT@LigNPs)上,以靶向幽门螺杆菌细胞膜。丙酮具有较强的氢键能力,能使不同分子量的木质素聚集在一起,增加酚羟基的浓度。透射电子显微镜(TEM)、动态光散射(DLS)、紫外-可见光谱(UV-vis)、傅里叶变换红外光谱(FTIR)和热重分析(TGA)表明,在反溶剂过程中调节水溶剂比和搅拌速率可制得形状规则、粒径为86.5±0.41 ~ 354.6±3.44 nm的均匀球形LigNPs。EGT@LigNPs在48小时内持续释放药物,并表现出优异的抗氧化和抗h。与未加载的LigNPs和纯EGT相比。此外,EGT@LigNPs与正常a成纤维细胞系(L929)表现出良好的生物相容性,增强了幽门螺杆菌的代谢活性和细胞内蛋白释放。利用透射电镜(TEM)、扫描电镜(SEM)和共聚焦激光扫描显微镜(CLSM)进行的生物成像研究表明,EGT@LigNPs粘附在细菌表面,破坏细胞膜的稳定性。这些发现表明EGT@LigNPs为治疗幽门螺杆菌引起的胃感染提供了一种无毒的口服药物输送系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrasonic nanoengineering of biocompatible lignin-ergothioneine hybrids for targeted membrane disruption and sustained oral eradication of Helicobacter pylori
Helicobacter pylori (H. pylori) is a gram-negative, spiral-shaped bacterium that infects approximately 4.4 billion people globally. In this study, a novel method combining high-intensity ultrasonic solvent treatment and homogenization was employed to functionalize and nano-transform lignin nanoparticles (LigNPs) for grafting ergothioneine (EGT), a natural hydrophobic antioxidant, onto LigNPs (EGT@LigNPs) to target the H. pylori cell membrane. Acetone, with its strong hydrogen bonding capacity, was found to aggregate lignin of various molecular weights and increase the concentration of phenolic hydroxyl groups. Transmission electron microscopy (TEM), dynamic light scattering (DLS), UV–vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA) revealed that LigNPs with a regular spherical shape and uniform size 86.5 ± 0.41 to 354.6 ± 3.44 nm could be produced by adjusting the water/solvent ratio and stirring rate during the antisolvent process. EGT@LigNPs demonstrated sustained drug release over 48 h and exhibited superior antioxidant and anti-H. pylori activities compared to unloaded LigNPs and pure EGT. Additionally, EGT@LigNPs showed excellent biocompatibility with normal a fibroblast cell line (L929), enhancing metabolic activity and intracellular protein release in H. pylori. Bioimaging studies using TEM, scanning electron microscopy (SEM), and confocal laser scanning microscopy (CLSM) indicated that EGT@LigNPs adhered to the bacterial surface and destabilized and disrupted the cell membranes. These findings suggest that EGT@LigNPs hold novel a non-toxic oral drug delivery system for treating gastric infections caused by H. pylori.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


