基于活MOG-IgG细胞的检测:流式细胞仪的比较和高灵敏度全谱流式细胞仪的诊断验证
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
MOG抗体相关疾病(MOGAD)的诊断依赖于MOG抗体(MOG- igg)的血清阳性。基于活细胞的检测(CBA)是金标准。尽管流式细胞术活体cba具有很高的真实世界灵敏度,但其全球实施和诊断部署受到“内部”设计和定制优化观念的挑战。在此,我们比较了流动活MOG-IgG CBA在研究和诊断实验室的各种细胞仪上的分析稳健性。流式活cba在三台常规(Fortessa, BDLSRII, Gallios)和两台光谱细胞仪(Aurora, ID7000)上进行。用中位荧光强度(MFI)计算MOG-IgG滴度,测定测定内、间精密度(CV%)和血清状态。目前用于检测的Fortessa上的MFI检测范围明显低于光谱ID7000(4.75倍,p = 0.04)和Aurora(12倍,p = 0.0001),尽管所有MFI都相关(p < 0.0001; R2 = 0.99)。有趣的是,高检测范围归因于技术,而不是实验室环境(p > 0.05)。在不同的细胞仪中,测定内和测定间的精密度相似。ID7000和Fortessa变异最小,cv内变异率分别为4.6%和6.8%,cv间变异率分别为15.7%。无论使用什么分析软件(p < 0.0001, R2 = 0.98),所有细胞仪的FACS比率(目前报道为MOG-IgG滴度)与mfi具有可比性和相关性(p < 0.001; R2≤0.99)。所有血清状态高度一致(κ = 1)。我们的研究结果表明,流动活cba可以在诊断实验室通过一系列流式细胞仪进行验证,具有高再现性和可重复性。特别是,光谱流式细胞术的优异分析性能有力地支持了该技术用于诊断目的的概念验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
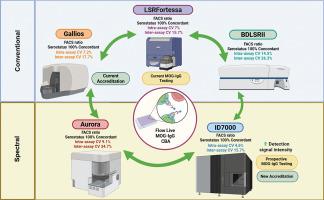
Live MOG-IgG cell-based assay: Comparison across flow cytometers and diagnostic validation on high-sensitivity full spectrum flow cytometry
MOG antibody-associated disease (MOGAD) diagnosis rests on seropositivity for MOG antibody (MOG-IgG). Live cell-based assays (CBA) are gold standards. Although flow cytometry live CBAs have high real-world sensitivity, their global implementation and diagnostic deployment have been challenged by perceptions of “in-house” design and custom optimization. Herein, we compared the analytical robustness of flow live MOG-IgG CBA across various cytometers in both research and diagnostic laboratories. Flow live CBAs were performed on three conventional (Fortessa, BDLSRII, Gallios), and two spectral cytometers (Aurora, ID7000). MOG-IgG titers were calculated by median fluorescence intensity (MFI), and intra- and inter assay precisions (CV%) and serostatuses were determined. The MFI detection range on Fortessa, currently used for testing, was significantly lower than spectral ID7000 (4.75-fold, p = 0.04) and Aurora (12-fold, p = 0.0001), albeit all MFIs correlated (p < 0.0001; R2 = 0.99). Interestingly, the high detection range was attributed to technology, not laboratory environments (p > 0.05). Intra- and inter-assay precisions were similar across cytometers. ID7000 and Fortessa had the lowest variation, with 4.6 % and 6.8 % intra-CV, respectively, and 15.7 % inter-CV. FACS ratio, currently reported as MOG-IgG titre, and MFIs were comparable and correlated for all cytometers (p < 0.001; R2 ≤ 0.99), regardless of the analysis software (p < 0.0001, R2 = 0.98). All serostatuses were highly concordant (κ = 1). Our results demonstrate that flow live CBAs can be validated in diagnostic laboratories across a range of flow cytometers with high reproducibility and repeatability. In particular, excellent assay performance on spectral flow cytometry strongly supports the proof-of-concept use of this technology for diagnostic purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


