AS-IV-HP-β-CD分子胶囊通过抑制NLRP3炎症小体介导的神经炎症,减轻mptp诱导PD小鼠的运动功能障碍和DA神经元丢失
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
本研究采用分子模拟对接技术,确定最适合包封黄芪甲苷(Astragaloside IV, AS-IV)的宿主分子。采用研磨法将AS-IV的疏水性基团包封在HP-β-CD的内腔中,制备AS-IV-HP-β-CD分子胶囊。采用扫描电镜、傅里叶变换红外光谱和液体核磁共振光谱对其包封行为进行了表征。发现AS-IV包封在HP-β-CD腔内,包封后呈层状结构。含量测定结果表明,与AS-IV相比,AS-IV- hp -β-CD在体外的溶解度和溶出率显著提高。药理学试验表明,口服AS-IV-HP-β-CD可有效改善mptp诱导的帕金森病小鼠的运动功能障碍,减轻多巴胺能(DA) th阳性神经元的损失,降低黑质致密部炎症因子IL-1β和IL-18的表达。同时,AS-IV-HP-β-CD干预抑制NLRP3炎性小体的表达,同时TLR4/MyD88/NF-κB通路蛋白的表达降低。该研究解决了AS-IV的水溶性和溶出率差的问题,为开发AS-IV的配方奠定了基础。同时,新的数据进一步支持了as - iv作为PD辅助治疗的有效膳食组合的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
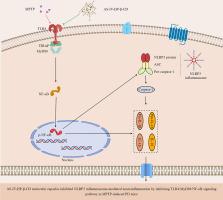
AS-IV-HP-β-CD molecular capsules alleviated motor dysfunction and DA neuronal loss by inhibiting NLRP3 inflammasome-mediated neuroinflammation in MPTP-induced PD mice
This study employed molecular simulation docking technology to identify the most suitable host molecules for encapsulating Astragaloside IV (AS-IV). AS-IV-HP-β-CD molecular capsules were prepared by encapsulating the hydrophobic groups of AS-IV in the inner cavity of HP-β-CD through the grinding method. The encapsulation behaviours were characterized by scanning electron microscopy, Fourier transform infrared spectroscopy, and liquid nuclear magnetic resonance spectroscopy. It was found that AS-IV was encapsulated inside the HP-β-CD cavity and presented a layered structure after encapsulation. Content determination showed that the solubility and dissolution rate of AS-IV-HP-β-CD in vitro were significantly increased compared to AS-IV. Pharmacodynamic tests demonstrated that oral administration of AS-IV-HP-β-CD effectively ameliorated motor dysfunction, alleviated dopaminergic (DA) TH-positive neuronal loss, and reduced the expression of inflammatory cytokines IL-1β and IL-18 in the substantia nigra pars compacta of MPTP-induced Parkinson's disease mice. Simultaneously, the AS-IV-HP-β-CD intervention inhibited the expression of the NLRP3 inflammasome, accompanied by a decrease in the expression of TLR4/MyD88/NF-κB pathway proteins. This study addressed the issues of poor water solubility and dissolution rate, laying the groundwork for developing AS-IV formulations. Meanwhile, new data further support the potential of AS-IV as an effective dietary combination for adjuvant therapy of PD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


