甜菊苷V:分子机制和治疗应用
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
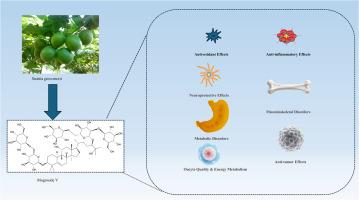
罗汉果苷V (mogroside V, MV)是罗汉果中主要的生物活性化合物,对氧化应激、炎症、骨质疏松、糖尿病、肥胖和神经系统损伤具有显著的治疗作用。目前,缺乏通过核心信号通路解释其治疗机制的综合综述。目的系统整理和总结近年来MV的分子机制及其治疗潜力。方法通过检索PubMed、Web of Science、谷歌Scholar等数据库发表的相关文献,结合京都基因基因组百科(KEGG)通路分析,探讨MV对上述疾病的作用机制。结果smv通过关键信号级联介导其多方面的生物学功能。抗炎作用通过Janus激酶/信号转导和转录激活因子(JAK/STAT)、蛋白激酶B/磷脂酰肌醇3-激酶/雷帕霉素机制靶点(PI3K/Akt/mTOR)、toll样受体4 (TLR4)和内质网应激(ERS)-未折叠蛋白反应(ERS- upr)通路促进。在抗氧化防御中,MV主要参与核(红细胞因子2相关因子2/血红素加氧酶1)Nrf2/HO-1、PI3K/Akt和sirtuin (SIRT)途径。此外,MV通过靶向amp激活的蛋白激酶(AMPK)途径调节代谢并提供治疗潜力,在肥胖中,调节PI3K/Akt/葡萄糖转运蛋白2(GLUT2)轴用于糖尿病管理,激活牛磺酸上调基因1 (TUG1)途径以促进成骨。结论smv是一种很有前途的多靶点治疗药物。进一步研究生物利用度优化和临床转化是必要的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mogroside V: Molecular mechanisms and therapeutic applications
Background
Mogroside V (MV) constitutes the primary bioactive compound within Siraitia grosvenorii and has significant therapeutic effects on oxidative stress, inflammation, osteoporosis, diabetes, obesity, and nervous system damage. Presently, there exists a shortage of comprehensive reviews explaining its therapeutic mechanisms through core signaling pathways.
Aim
To systematically organize and summarize recent advances MV's molecular mechanisms and its therapeutic potential.
Methods
The potential mechanisms of MV on the above diseases were explored by searching relevant literature published in databases such as PubMed, Web of Science, and Google Scholar, combined with the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
Results
MV mediates its multifaceted biological functions via key signaling cascades. Anti-inflammatory actions are facilitated through pathways of Janus kinase/signal transducer and activator of transcription (JAK/STAT), Protein kinase B/ phosphatidylinositol 3-kinase/mechanistic target of rapamycin (PI3K/Akt/mTOR), Toll-like receptor 4 (TLR4), and Endoplasmic reticulum stress (ERS)-unfolded protein response (ERS-UPR). For anti-oxidant defense, MV predominantly engages the Nuclear (factor erythroid 2-related factor 2/heme oxygenase 1) Nrf2/HO-1, PI3K/Akt, and sirtuin (SIRT) pathways. Furthermore, MV regulates metabolism and offers therapeutic potential by targeting the AMP-activated Protein Kinase (AMPK) pathway in obesity, modulating the PI3K/Akt/ glucose transporter type 2(GLUT2) axis for diabetes management, and activating the Taurine Upregulated Gene 1 (TUG1) pathway to enhance osteogenesis.
Conclusions
MV is a promising multi-target therapeutic candidate. Further studies on bioavailability optimization and clinical translation are warranted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


