Nd@g-C3N4 3-氟烷基化喹啉-2(1H)- 1的双功能光合作用和抗肿瘤活性
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文首次报道了在可见光和超声条件下,Nd@g-C3N4双功能光催化下与RfSO2Cl进行烯烃氟烷基异芳化反应。光生电子驱动的氟烷基自由基的还原产物与光生空穴驱动的氯自由基的氧化产物配对,使得光生载体被充分利用来形成键。广泛的n -杂芳烃、烯烃和RfSO2Cl在该反应中具有良好的相容性,可以获得具有不同结构特征的有价值的氟烷基化n -杂芳烃。采用CCK8法评价合成的氟烷基化n -杂环对胶质瘤261细胞的抗肿瘤作用。值得注意的是,化合物4aka显示出显著的疗效,其效力比广泛使用的化疗药物替莫唑胺高出约7倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
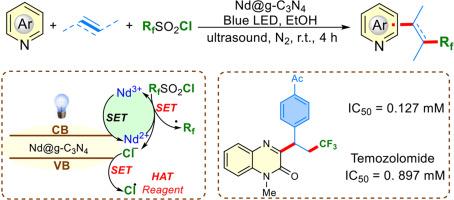
Nd@g-C3N4 dual-functional photosynthesis and antitumor activities of 3-fluoroalkylated quinoxalin-2(1H)-ones
Herein, the Nd@g-C3N4 dual-functional photocatalysis enabled fluoroalkylative heteroarylation of alkenes with RfSO2Cl under visible-light and ultrasound conditions was firstly reported. The photogenerated electron-driven reductive production of fluoroalkyl radical paired with photogenerated hole-driven oxidative production of chloride radical resulted in the full utilization of photogenerated carrier for bond formation. A wide range of N-heteroarenes, alkenes and RfSO2Cl, were well compatible for this reaction to access valuable fluoroalkylated N-heteroarenes with diverse structural features. The antitumor potential of synthesized fluoroalkylated N-heterocycles against Glioma 261 cells was evaluated by CCK8 assay. Notably, compound 4aka demonstrated remarkable efficacy, exhibiting approximately sevenfold greater potency than temozolomide, a widely used chemotherapeutic agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


