真菌菌丝对外界刺激的电信号检测
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
电信号是跨多种生物系统的细胞间通讯的重要机制。尽管有证据表明真菌菌丝体中存在电活动,但缺乏一种标准化的、可重复的检测这些信号的方法。在这项研究中,我们开发了一种新的方法,使用嵌入差分电极的印刷电路板来记录菌丝体的细胞外电压波动。通过结合法拉第笼和短时傅里叶变换分析,我们最小化了噪声并提取了相关的频率模式。我们的研究结果显示,电活动与真菌生长相关,随着杀菌剂处理的不同而变化。这些结果支持这些信号的生物学起源,表明它们在环境适应中起作用。该研究为进一步探索真菌电生理学提供了一个强有力的框架,对理解菌丝网络中的信号机制具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
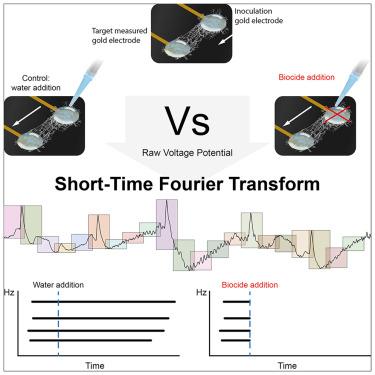
Detection of electrical signals in fungal mycelia in response to external stimuli
Electrical signaling is a crucial mechanism for intercellular communication across diverse biological systems. Despite evidence of electrical activity in fungal mycelia, a standardized, reproducible method for detecting these signals is lacking. In this study, we developed a novel approach using printed circuit boards with embedded differential electrodes to record extracellular voltage fluctuations in mycelium. By incorporating a Faraday cage and short-time Fourier transform analysis, we minimized noise and extracted relevant frequency patterns. Our findings revealed electrical activity correlated with fungal growth that varied with biocide treatments. The results support the biological origin of these signals, suggesting a role in environmental adaptation. This study provides a robust framework for further exploration of fungal electrophysiology, with implications for understanding signaling mechanisms in mycelial networks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


