自扩增和环状mRNA疫苗对SARS-CoV-2免疫原性和保护效果的比较
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
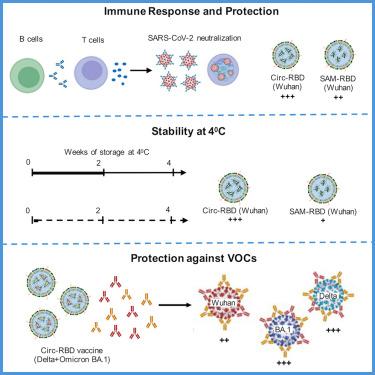
疫苗技术的最新进展使信使RNA (mRNA)疫苗成为人类使用的安全可靠的选择。传统上,mRNA疫苗是使用线性或自扩增mRNA (SAM)设计的,后者被认为是更好的。随后对环状mRNA (Circ-RNA)疫苗的研究证实了它们的有效性。在这里,我们比较了使用SARS-CoV-2-RBD(受体结合域)抗原的SAM-和Circ-RNA疫苗的疗效。SAM-RBD和Circ-RBD诱导的抗rbd IgG滴度和病毒中和抗体滴度相当。然而,后者诱导了更高的记忆T细胞反应。Circ-RBD疫苗在4℃下稳定4周。一种含有δ型和组粒型SARS-CoV-2变体Circ-RBD的二价疫苗能有效中和这些病毒。这些发现表明Circ-RNA- rbd是一种优秀的COVID-19候选疫苗,也为开发针对SARS-CoV-2或其他快速出现变体的病毒的二价Circ-RNA候选疫苗提供了平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comparison of immunogenicity and protection efficacy of self-amplifying and circular mRNA vaccines against SARS-CoV-2
Recent advances in vaccine technology have positioned messenger RNA (mRNA) vaccines as safe and reliable options for human use. Conventionally, mRNA vaccines were designed using linear or self-amplifying mRNA (SAM), the latter considered to be superior. Subsequent studies on Circular mRNA (Circ-RNA) vaccines proved their efficacy. Here, we compared the efficacy of SAM- and Circ-RNA vaccines using the SARS-CoV-2-RBD (receptor binding domain) antigen. Both SAM-RBD and Circ-RBD induced a comparable anti-RBD IgG titer and virus-neutralizing antibody titer. However, the latter induced a higher memory T cell response. The Circ-RBD vaccine is stable for 4 weeks at 4°C. A bivalent vaccine containing Circ-RBD of both delta and omicron SARS-CoV-2 variants potently neutralized these viruses. These findings demonstrate Circ-RNA-RBD as an excellent vaccine candidate against COVID-19 and also provide a platform for developing bivalent Circ-RNA vaccine candidates against SARS-CoV-2 or other viruses with rapidly emerging variants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


