超声治疗纳米铁霉素通过超声引爆ROS产生和铁中毒样死亡根除细菌生物膜感染
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
生物膜的形成对抗菌管理提出了严峻的挑战。虽然铁载体-抗生素缀合物(称为铁霉素)提供了一种很有希望的解决方案,但其功效本身受到抗生素耐药性的限制。为了超越这一障碍,我们开创了一种变革性的铁载体-声敏剂缀合物,通过儿茶酚铁载体与purpurin 18(一种声敏剂)的共价键连接。这种新型的结合物进一步与铁(III)离子自组装,形成了第一个无载体的纳米铁霉素——一种全在铁-铁载体上的声敏剂纳米平台。这种设计使超声波表示的活性氧(ROS)的产生和铁中毒样扩增成为可能。利用细菌特异性的铁载体摄取和ph反应性的组装/拆卸,纳米铁霉素能够精确递送和主动内化声敏剂进入细菌。该策略允许通过并发荧光/光声和磁共振成像实时定位感染。在超声照射下,声敏剂介导的声动力治疗和铁载体/铁增强的声芬顿催化的双重抗菌机制被刺激释放,协同触发爆炸性ROS爆发和强效的铁中毒样细菌死亡。结果,多重耐药生物膜诱导的化脓性炎小鼠被完全治愈。总的来说,这种一流的治疗性纳米红霉素集成了高靶向成像诊断、成本效益高但超高效的ROS生成和铁中毒样细菌杀死,为具有时空可控性的生物膜治疗建立了一种范式转换策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
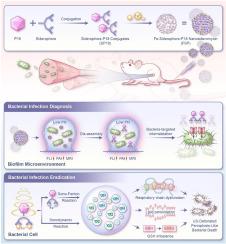
Sonotheranostic nanosideromycin eradicates bacterial biofilm infections via ultrasound-detonated ROS generation and ferroptosis-like death
Biofilm formation poses a severe challenge to antibacterial stewardship. While siderophore-antibiotic conjugates (termed as sideromycins) offer a promising solution, their efficacy is inherently limited by antibiotic resistance. To transcend this barrier, we pioneer a transformative siderophore-sonosensitizer conjugate through covalent linkage of a catechol siderophore to purpurin 18 (a sonosensitizer). This novel conjugate further self-assembles with iron(III) ions, forming the first-reported carrier-free nanosideromycin—an all-in-on iron-siderophore-sonosensitizer nanoplatform. This design enables ultrasound-denotated reactive oxygen species (ROS) generation and ferroptosis-like amplication. Capitalizing on bacteria-specific siderophore uptake and pH-responsive assembly/disassembly, the nanosideromycin enables precision delivery and active internalization of sonosensitizers into bacteria. This strategy permits real-time localization of infections via concurrent fluorescence/photoacoustic and magnetic resonance imaging. Upon ultrasound irradiation, dual antimicrobial mechanisms of sonosensitizer-mediated sonodynamic therapy and siderophore/iron-augmented sono-Fenton catalysis are stimuonously unleashed, synergistically tirggering an explosive ROS burst and potent ferroptosis-like bacterial death. As a result, mice with multidrug-resistant biofilm-induced pyomyositis were completely cured. Collectively, this first-in-class theranostic nanosideromycin integrates highly-targeted imaging diagnostics, cost-effective yet ultra-efficient ROS generation, and ferroptosis-like bacterial killing, establishing a paradigm-shifting strategy for biofilm therapy with spatiotemporal controllability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


