辅酶基弹性贴片作为铁离子捕获剂调节糖尿病口腔溃疡修复中的铁代谢
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
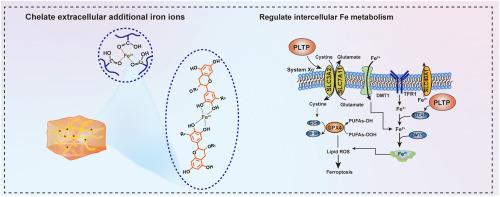
高糖和营养丰富条件下的铁代谢失衡是抑制糖尿病口腔溃疡愈合的关键因素。本研究证实了糖尿病口腔溃疡中广泛的铁积累和铁下垂的发生。为了解决这个问题,将硫辛酸(LA)和茶多酚(TP)这两种天然生物活性分子结合在一起,设计了一种口服贴剂(PLTP)。PLTP中丰富的羧基和羟基充当铁离子捕集器,吸附胞外铁,减少铁转运到细胞内,有效切断细胞内铁下垂的铁源。LA和TP在创面部位的原位释放,不仅通过调节细胞内Xc−/GPX4轴,增强抗氧化能力,上调SLC40A1的表达,下调DMT1的表达,帮助恢复细胞间铁稳态,还可以调节免疫微环境,进一步支持创面愈合。此外,PLTP在富铁溶液中剧烈搅拌24 h后,与口腔黏膜的粘附强度仍为14.32 kPa,在体内可在湿润动态的口腔中保持24 h以上的长时间粘附。这有效地保护溃疡部位免受细菌入侵和食物残渣污染。体内实验表明,PLTP显著加速糖尿病口腔溃疡愈合,第8天创面愈合率达到92.4%,优于商业壳聚糖贴片(53.7%)和抗上铁药物去铁氧(60.0%)。在体内进一步系统验证PLTP的潜在机制和治疗效果,希望为糖尿病组织修复铁代谢调控提供新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coenzyme-based elastomeric adhesive patch as iron ion capturer to regulate iron metabolism for diabetic oral ulcer repair
Iron metabolism imbalance under high-glucose and nutrient-rich conditions is a key factor inhibiting the healing of diabetic oral ulcers. This study confirms the extensive iron accumulation and ferroptosis occurrence in diabetic oral ulcers. To address this, an oral patch (PLTP) is designed by combining two natural bioactive molecules of lipoic acid (LA) and tea polyphenols (TP). The abundant carboxyl and hydroxyl groups in PLTP act as iron ion capturers, adsorbing extracellular iron, reducing iron transport into cells, and effectively cutting off the iron source for intracellular ferroptosis. The in-situ release of LA and TP at wound site not only aids in restoring intercellular iron homeostasis via regulating the intracellular Xc−/GPX4 axis, enhancing antioxidant capacity, upregulating the expression of SLC40A1, and downregulating the expression of DMT1, but also modulates the immune microenvironment, further supporting wound healing. In addition, PLTP exhibits a robust adhesion strength of 14.32 kPa to oral mucosa even after 24 h of vigorous stirring in an iron-rich solution in vitro, and maintains prolonged adhesion in the moist and dynamic oral cavity for over 24 h in vivo. This effectively protects ulcer sites from bacterial invasion and food debris contamination. In vivo experiments show that PLTP significantly accelerates diabetic oral ulcer healing, achieving 92.4 % wound closure by day 8, outperforming both commercial chitosan patches (53.7 %) and the anti-ferroptosis drug Deferasirox (60.0 %). The underlying mechanisms and therapeutic effects of PLTP are further systematically validated in vivo, hoping to provide new insights into iron metabolism regulation for tissue repair in diabetes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


