脂质代谢重编程和免疫抑制前哨淋巴结的双重靶向增强了三阴性乳腺癌的抗转移治疗
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
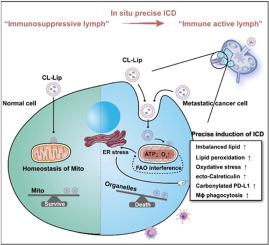
诱导癌细胞免疫原性细胞死亡(ICD)是一种很有前景的免疫治疗方法,然而,由于脂肪酸的代谢,传统ICD诱导剂在前哨淋巴结(SLN)中的氧化应激缓解和代谢可塑性增强限制了其免疫刺激作用。本文设计了一种具有生物相容性的绿色脂质体CL-Lip,该脂质体不仅可以在原发和转移性4T1细胞中选择性诱导ICD,还可以缓解侵袭性SLN的免疫抑制。CL-Lip由亚油酸和过氧化氢酶修饰的工程脂质体组成,协同触发ICD,刺激脂质过氧化、PD-L1羰基化,有效促进树突状细胞成熟和T细胞分化。过氧化氢酶也下调了SLN的缺氧水平。通过细胞实验和转录组分析,证明CL-Lip诱导ICD是由yap依赖性脂肪酸氧化(FAO)干扰引起的ROS生成介导的。转录组分析显示,工程化的CL-Lip减少yap依赖的FAO通路,有效地拮抗代谢灵活性,从而选择性地触发原发性和转移性4T1细胞中代谢死亡相关的ICD过程。在动物实验中,这种很少报道的代谢驱动的ICD途径不仅显著减少转移灶,而且诱导SLN的“冷到热”重塑,导致原位肿瘤疫苗的形成。这些发现对开发下一代ICD诱导剂和免疫治疗方法具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dual targeting of lipid metabolic reprogramming and immunosuppressive sentinel lymph nodes potentiates anti-metastatic therapy for triple negative breast cancer
Inducing immunogenic cell death (ICD) in cancer cells provides a promising approach in immunotherapy, however, oxidative stress relief and metabolic plasticity enhancement limit the immune-stimulating effect of traditional ICD inducer in sentinel lymph nodes (SLN), owing to the metabolism of fatty acids. In this article, a biocompatible green liposome CL-Lip was designed to not only selectively induce ICD in primary and metastatic 4T1 cells, but also relieved the immunosuppression in invasive SLN. CL-Lip is composed of engineered liposomes modified with linoleic acid and catalase, which synergistically trigger ICD, stimulate lipid peroxidation, PD-L1 carbonylation and effectively promote the maturation of dendritic cells and T cell differentiation. Moreover, catalase also downregulated the hypoxia level in SLN. Through cellular experiments and transcriptome analysis, it is proved that the ICD induction via CL-Lip is mediated by ROS generation, resulting from the YAP-dependent fatty acid oxidation (FAO) interference. Transcriptome analysis revealed that engineered CL-Lip diminishes YAP-dependent FAO pathway and effectively antagonizes the metabolic flexibility, thereby selectively triggering the metabolic dead-associated ICD process in both primary and metastatic 4T1 cells. In animal experiments, this little reported metabolic-driven ICD route not only significantly reduces metastatic foci, but also induces a “cold-to-hot” remodeling of SLN, resulting in the formation of a in situ tumor vaccine. These findings hold great significance for the development of next-generation ICD inducers and immunotherapy approaches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


