二价mRNA疫苗增强剂对K18-hACE2小鼠的XBB.1.5免疫比突破感染更有效
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
SARS-CoV-2 Omicron亚变体的迅速出现,包括高度免疫逃避的XBB.1.5谱系,继续挑战疫苗的有效性。我们评估了K18-hACE2小鼠在接受两剂或三剂二价mRNA疫苗(编码武汉-1和Omicron BA.4/5刺突)或两剂后鼻内暴露XBB.1.5的免疫反应和对XBB.1.5攻击的保护作用。与突破感染相比,二价疫苗同源增强能诱导更强的中和抗体和细胞免疫应答。单独的两剂系列可以保护小鼠免受严重疾病的侵害,但可以进一步增强保护,减少肺部病毒RNA和炎症。虽然鼻内暴露后的生产性感染尚未得到证实,但增强的反应和病毒控制表明免疫记忆的重新激活。总体而言,在幼稚小鼠模型中,二价疫苗的同源增强增强了跨变体免疫,并对XBB.1.5具有保护作用,支持更新疫苗在提高免疫广度和对新出现的SARS-CoV-2变体的保护方面的效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
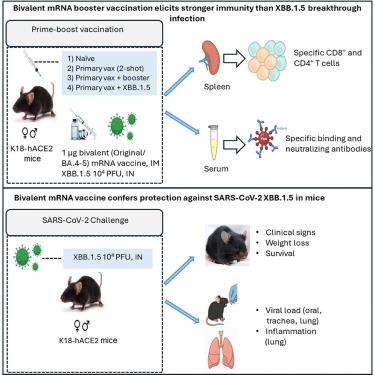
Bivalent mRNA vaccine booster enhances immunity against XBB.1.5 more effectively than breakthrough infection in K18-hACE2 mice
The rapid emergence of SARS-CoV-2 Omicron subvariants, including the highly immune-evasive XBB.1.5 lineage, continues to challenge vaccine effectiveness. We evaluated immune responses and protection against XBB.1.5 challenge in K18-hACE2 mice receiving two or three doses of a bivalent mRNA vaccine (encoding Wuhan-1 and Omicron BA.4/5 spike) or two doses followed by intranasal XBB.1.5 exposure. Homologous boosting with bivalent vaccine induced stronger neutralizing antibody and cellular immune responses than breakthrough infection against the antigenically distant XBB.1.5 variant. A two-dose series alone protected mice from severe disease, but boosting further enhanced protection, reducing lung viral RNA and inflammation. Although productive infection following intranasal exposure was not confirmed, enhanced responses and viral control suggest reactivation of immune memory. Overall, homologous boosting with the bivalent vaccine enhances cross-variant immunity and protects against XBB.1.5 in a naive mouse model, supporting the utility of updated vaccines in improving immune breadth and protection against emerging SARS-CoV-2 variants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


