m1样巨噬细胞通过P2X4受体调控T细胞在结直肠癌中的浸润
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
尽管肿瘤相关巨噬细胞(tam)在结直肠癌(CRC)中起着关键的免疫调节作用,但其极化的机制尚不清楚。本研究确定P2X4受体(P2X4R)是m1样极化的关键介质。在使用CRC细胞条件培养基的受控体外系统中,我们通过免疫荧光和线粒体检测观察到p2x4r介导的钙内流和随后的线粒体功能障碍。这种功能障碍导致线粒体DNA释放并随后激活cGAS-STING-IFNB1通路,驱动tam的m1样极化。流式细胞术显示,表达p2x4r的tam不仅能增强体外CD8+ T细胞的存活和细胞毒性,还能增强同基因CRC小鼠模型中的T细胞反应。临床上,P2X4在结直肠癌组织中的表达降低与预后较差相关。总之,这些发现确定了P2X4R是m1样TAM极化的关键调节因子,代表了TAM重编程和抑制CRC进展的有希望的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
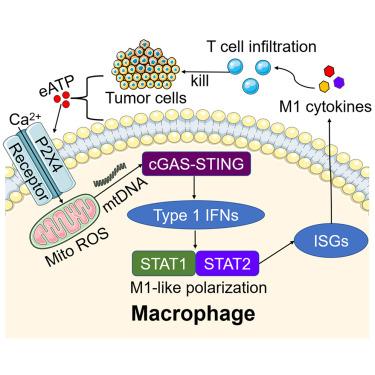
M1-like macrophages regulate T cell infiltration in colorectal cancer through P2X4 receptor
Although tumor-associated macrophages (TAMs) play a critical immunomodulatory role in colorectal cancer (CRC), the mechanisms underlying their polarization remain unclear. This study identifies the P2X4 receptor (P2X4R) as a crucial mediator of M1-like polarization. During TAM induction in a controlled in vitro system using CRC cell-conditioned medium, we observed P2X4R-mediated calcium influx and subsequent mitochondrial dysfunction through immunofluorescence and mitochondrial assays. This dysfunction led to mitochondrial DNA release and subsequent activation of the cGAS-STING-IFNB1 pathway, driving M1-like polarization of TAMs. Flow cytometry demonstrated that P2X4R-expressing TAMs not only enhanced CD8+ T cell survival and cytotoxicity in vitro but also augmented T cell responses in a syngeneic CRC mouse model. Clinically, reduced P2X4 expression in CRC tissues correlated with poorer prognosis. In conclusion, these findings identify the P2X4R as a key regulator of M1-like TAM polarization, representing a promising target to reprogram TAMs and suppress CRC progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


