掺钽β-TCP多孔生物陶瓷支架:体外和体内生物降解和骨诱导性能的评价
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-18
DOI:10.1016/j.bioadv.2025.214517
引用次数: 0
摘要
开发具有平衡的力学性能、成骨活性和可控制降解的生物可降解骨替代品仍然是骨组织工程的关键挑战。本研究采用微波超声辅助的方法合成了原位掺杂ta掺杂的β-磷酸三钙(β-TCP)粉末,并利用该材料制备了多孔生物陶瓷支架。系统评价了Ta掺杂浓度对支架的理化性能、体外和体内生物安全性以及成骨性能的影响。结果表明,Ta5+的掺入显著改善了结构稳定性,抗压强度从5.65 MPa(纯β-TCP)提高到9.84 MPa (2.5% Ta组),同时保持了高孔隙率(65.98%)。体外实验表明,该支架具有良好的细胞相容性,细胞存活率超过90%,其中2.5% Ta组对成骨分化的促进作用最为显著,表现为ALP活性和钙沉积的增强。体内实验进一步证实了更好的骨再生性能,2.5% Ta组在12周时达到42.29%的骨体积分数(BV/TV),显著高于纯β-TCP组的22.27%。这些发现强调了Ta在同时改善机械完整性、生物降解动力学和骨诱导性方面的作用,建立了2.5% Ta掺杂β-TCP作为临床骨再生应用的非常有前途的支架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
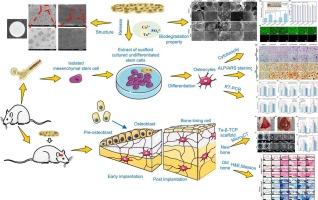
Tantalum-doped β-TCP porous bioceramic scaffolds: In vitro and in vivo evaluation of biodegradation and osteoinductive properties
The development of biodegradable bone substitutes with balanced mechanical properties, osteogenic activity, and controlled degradation remains a critical challenge in bone tissue engineering. In this study, in situ doping Ta-doped β-tricalcium phosphate(β-TCP) powders were synthesized via a microwave-ultrasound-assisted method, and porous bioceramic scaffolds were prepared from this material. The effects of Ta doping concentration on the scaffolds' physicochemical properties, in vitro and in vivo biological safety and osteogenic properties of the scaffolds were systematically evaluated. The results showed that Ta5+ incorporation significantly improved structural stability and enhanced compressive strength from 5.65 MPa (pure β-TCP) to 9.84 MPa (2.5 % Ta group), while maintaining high porosity (65.98 %). In vitro, the scaffolds showed excellent cytocompatibility with cell viability exceeding 90 %, and the 2.5 % Ta group exhibited the most substantial promotion of osteogenic differentiation, as evidenced by enhanced ALP activity and calcium deposition. In vivo experiments further confirmed superior bone regenerative performance, with the 2.5 % Ta group achieving a bone volume fraction (BV/TV) of 42.29 % at 12 weeks, significantly higher than the 22.27 % observed in pure β-TCP. These findings underscore the role of Ta in simultaneously improving mechanical integrity, biodegradation kinetics, and osteoinductivity, establishing 2.5 % Ta-doped β-TCP as a highly promising scaffold for clinical bone regeneration applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


