硒纳米颗粒通过激活PRDX6/GPX4通路抑制铁下沉,减轻人工关节金属假体的钴毒性
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
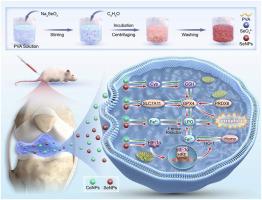
金属关节假体的长期植入导致钴纳米颗粒(CoNPs)的释放,导致局部甚至全身毒性反应,对患者健康构成威胁。先前的研究表明,conps诱导的细胞毒性可能与过度氧化应激和铁下垂有关。硒纳米粒子(SeNPs)以其抗铁腐性而闻名,具有作为金属植入物表面涂层的潜力。本研究旨在探讨SeNPs在抑制铁下沉和减轻钴致毒性中的作用和机制,从而为改善金属假体的材料性能提供机制依据。我们首先对髋关节翻修手术患者的组织样本进行了分子分析,发现了铁中毒相关信号通路的激活。然后,我们合成了能够被骨髓来源的基质细胞(BMSCs)有效内化的SeNPs。在体外,400 μM CoNPs通过抑制SLC7A11/GPX4轴和激活HIF-1α/HO-1信号通路诱导BMSCs铁凋亡的标志特征。相反,40 μM SeNPs处理上调PRDX6和GPX4,从而减轻铁凋亡并保持细胞活力。最后,在小鼠膝关节关节内注射SeNPs可显著减轻conps引起的局部毒性反应,包括滑膜增生和软骨破坏。总的来说,这项研究为conps诱导的毒性背后的铁中毒依赖机制提供了新的见解,并强调了SeNPs作为解毒剂的治疗潜力。这些发现为靶向解毒策略提供了机制基础,并为改进金属假体材料的发展提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selenium nanoparticles alleviate cobalt toxicity in artificial joint metal prostheses by inhibiting ferroptosis through activation of the PRDX6/GPX4 pathway
The long-term implantation of metallic joint prostheses results in the release of cobalt nanoparticles (CoNPs), leading to local and even systemic toxic reactions that pose risks to patient health. Previous studies have suggested that CoNPs-induced cytotoxicity may be associated with excessive oxidative stress and ferroptosis. Selenium nanoparticles (SeNPs), known for their anti-ferroptotic properties, have potential as surface coatings for metal implants. This study aims to investigate the role and mechanisms of SeNPs in inhibiting ferroptosis and mitigating cobalt-induced toxicity, thereby offering a mechanistic rationale for improving the material properties of metal prostheses. We first conducted molecular analyses on tissue samples from patients undergoing hip joint revision surgery, which revealed activation of ferroptosis-related signaling pathways. We then synthesized SeNPs capable of effective internalization by bone marrow-derived stromal cells (BMSCs). In vitro, 400 μM CoNPs induced hallmark features of ferroptosis in BMSCs by suppressing the SLC7A11/GPX4 axis and activating the HIF-1α/HO-1 signaling pathway. In contrast, treatment with 40 μM SeNPs upregulated PRDX6 and GPX4, thereby attenuating ferroptosis and preserving cell viability. Finally, intra-articular injection of SeNPs into mouse knee joints significantly alleviated CoNPs-induced local toxic responses, including synovial hyperplasia and cartilage destruction. Overall, this study provides novel insights into the ferroptosis-dependent mechanisms underlying CoNPs-induced toxicity and highlights the therapeutic potential of SeNPs as a detoxifying agent. These findings offer a mechanistic foundation for targeted detoxification strategies and inform the development of improved metal prosthetic materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


