超声激活压电支架靶向双Ca2+/NF-κB信号通路,协调免疫调节和骨生成,加速骨再生
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由于炎症失调和成骨功能受损,临界大小的骨缺损无法愈合。为了克服静态生物材料的局限性,我们设计了3d打印的聚己内酯支架,其中包含聚多巴胺包被的BaTiO3/β-TCP纳米颗粒(10% PBT),用于动态超声(US)激活。体外/体内综合分析显示,us激活的压电触发了两个协同途径:(1)电压门控钙通道(VGCC)依赖的Ca2+内流特异性地在成骨细胞中(由ω-Hexatoxin-HV1A抑制),激活Ca2+/NFAT信号传导和直接矿化;(2)抑制巨噬细胞NF-κB p65磷酸化和核易位,驱动抗炎M2极化。关键是,M2巨噬细胞分泌促再生因子(BMP-2, VEGF),通过旁分泌信号增强成骨细胞分化和血管生成。在大鼠的严重缺陷中,10% PBT + US使骨体积(BV/TV)增加3倍,胶原成熟,CD31+血管增加,Runx2/BMP-2表达升高。这项工作揭示了靶向电免疫工程的一个范例:无线US动态地协调VGCC介导的Ca2+骨诱导和NF-κB抑制M2极化与BMP-2/VEGF分泌,为精确骨再生提供有效的,无生长因子的Ca2+和炎症信号的时空控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
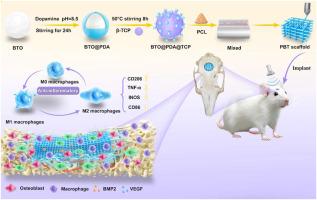
Ultrasound-activated piezoelectric scaffolds target dual Ca2+/NF-κB signaling pathways to orchestrate immunomodulation and osteogenesis for accelerated bone regeneration
Critical-sized bone defects fail to heal due to dysregulated inflammation and impaired osteogenesis. To overcome the limitations of static biomaterials, we engineered 3D-printed polycaprolactone scaffolds incorporating polydopamine coated BaTiO3/β-TCP nanoparticles (10 %PBT) for dynamic ultrasound (US) activation. Integrated in vitro/in vivo analyses revealed that US-activated piezoelectricity triggered two synergistic pathways: (1) Voltage gated calcium channel (VGCC) dependent Ca2+ influx specifically in osteoblasts (inhibited by ω-Hexatoxin-HV1A), activating Ca2+/NFAT signaling and direct mineralization; and (2) Suppression of NF-κB p65 phosphorylation and nuclear translocation in macrophages, driving anti-inflammatory M2 polarization. Crucially, M2 macrophages secreted pro-regenerative factors (BMP-2, VEGF), enhancing osteoblast differentiation and angiogenesis via paracrine signaling. In rat critical defects, 10 %PBT + US achieved 3 fold higher bone volume (BV/TV), mature collagen, increased CD31+ vessels, and elevated Runx2/BMP-2 expression. This work unveils a paradigm of targeted electro immunoengineering: wireless US dynamically orchestrates VGCC mediated Ca2+ osteoinduction and NF-κB inhibited M2 polarization with BMP-2/VEGF secretion, providing potent, growth factor free spatiotemporal control of Ca2+ and inflammatory signaling for precision bone regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


