肌球蛋白-肌动蛋白模式将基质硬度与gfat2透明质酸代谢联系起来
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
虽然失调的细胞外基质沉积和硬化被认为是驱动肿瘤进展的因素,但乳腺癌细胞可以持续存在、复发和转移到软微环境。肿瘤细胞适应软基质的不同策略仍有待进一步探索。在这里,我们报道乳腺肿瘤细胞利用软基质激活gfat2介导的透明质酸代谢,可以调节巨噬细胞。这一过程是通过NF-κB和XBP1s的核易位增强GFAT2表达的上调,加上GFAT活性的升高和随后通过抑制AMPKα磷酸化产生透明质酸。在机制上,GFAT2的表达和活性是由肌球蛋白和f -肌动蛋白的细胞总水平共同调节的。更具体地说,ROCK-Rac1平衡调节NF-κB-XBP1s-GFAT2信号轴,可以调节活性肌球蛋白的皮质与胞质比例和皮质f -肌动蛋白的周向排列。此外,我们的计算机模型验证了肌球蛋白的空间模式直接调节皮层f -肌动蛋白排列的方向。这些发现在基质顺应性和有害介质(nf -κB/XBP1s、GFAT和透明质酸)之间建立了一种新的机械代谢联系,揭示了肿瘤细胞在软壁龛中采用的潜在微环境重编程策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
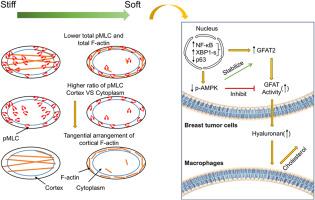
Myosin-actin pattern links matrix stiffness to GFAT2-hyaluronan metabolism
While dysregulated extracellular matrix deposition and stiffening are known to drive tumor progression, breast cancer cells can persist, relapse, and metastasize to soft microenvironments. The distinct strategies that tumor cells adapted to soft matrices remain to be further explored. Here, we report that breast tumor cells exploit soft matrices to activate GFAT2-mediated hyaluronan metabolism that can modulate macrophages. This process is driven by the upregulation of GFAT2 expression through enhanced nuclear translocation of NF-κB and XBP1s, coupled with elevated GFAT activity and subsequent hyaluronan production via suppressed AMPKα phosphorylation. Mechanistically, the expression and activity of GFAT2 are jointly modulated by the total cellular levels of myosin and F-actin. More specifically, the ROCK-Rac1 balance, which can regulate both the cortical-to-cytoplasmic ratio of active myosin and the circumferential arrangement of cortical F-actin, mediates the NF-κB-XBP1s-GFAT2 signaling axis. Furthermore, our in silico modeling validates that the spatial pattern of myosin directly regulates the orientation of cortical F-actin arrangement. These findings establish a novel mechano-metabolic link between matrix compliance and detrimental mediators—NF-κB/XBP1s, GFAT, and hyaluronan—uncovering a potential microenvironmental reprogramming strategy adopted by tumor cells in soft niches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


