胆红素聚合物纳米载体能够通过nrf2介导的铁凋亡/细胞凋亡抑制靶向法瑞罗递送青光眼神经保护
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
青光眼是不可逆失明的主要原因,其特点是视网膜神经节细胞(RGC)进行性变性,治疗选择有限。天然类黄酮法罗醇通过抗氧化和抗炎作用具有神经保护潜力,但其生物利用度和光降解性差限制了其治疗应用。本研究探讨了法瑞罗的神经保护机制,并开发了一种优化的纳米配方,以提高疗效。体外氧葡萄糖剥夺/再灌注(OGD/R)和体内缺血再灌注(IR)模型表明,法瑞罗可显著改善RGC存活和视觉功能。机制研究表明,法瑞罗激活核因子红细胞2相关因子2 (Nrf2)抗氧化途径,减少活性氧(ROS)积累,调节铁死亡关键标志物谷胱甘肽过氧化物酶4 (GPX4)和酰基辅酶a合成酶长链家族成员4 (ACSL4),从而抑制RGC细胞凋亡。为了克服递送限制,研究人员开发了装载法罗醇的胆红素纳米颗粒(FB-NPs),在青光眼损伤模型中显示出增强的稳定性和神经保护作用。这些发现确定了Nrf2/铁下垂/凋亡轴作为一种新的治疗靶点,并提出了一种有效的青光眼纳米递送策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
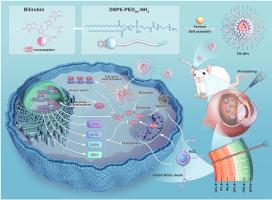
Bilirubin-polymer nanocarriers enable targeted farrerol delivery for glaucoma neuroprotection via Nrf2-mediated ferroptosis/apoptosis inhibition
Glaucoma, a leading cause of irreversible blindness, is characterized by progressive retinal ganglion cell (RGC) degeneration with limited treatment options. The natural flavonoid farrerol exhibits neuroprotective potential through antioxidative and anti-inflammatory effects, but its therapeutic application is limited by poor bioavailability and photodegradation. This study investigates farrerol's neuroprotective mechanisms and develops an optimized nanoformulation for enhanced efficacy. In vitro oxygen-glucose deprivation/reperfusion (OGD/R) and in vivo ischemia-reperfusion (IR) models demonstrate that farrerol significantly improves RGC survival and visual function. Mechanistic studies reveal that farrerol activates the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway, reduces reactive oxygen species (ROS) accumulation, and modulates key ferroptosis markers (glutathione peroxidase 4 (GPX4) and acyl-CoA synthetase long-chain family member 4 (ACSL4)), thereby inhibiting RGC apoptosis. To overcome delivery limitations, farrerol-loaded bilirubin nanoparticles (FB-NPs) are developed, showing enhanced stability and neuroprotective effects in glaucomatous injury models. These findings identify the Nrf2/ferroptosis/apoptosis axis as a novel therapeutic target and present an effective nanodelivery strategy for glaucoma treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


