协同双重化学物理FeCu-MOF支架与PEMF刺激驱动血管生成-成骨耦合骨再生
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
有效修复大面积骨缺损需要同时进行骨生成和血管生成,这是传统生物材料面临的一个重大挑战,通常受限于次优结构设计和无法提供时空可控的生物活性线索。在这里,我们报告了一种新的化学物理双响应系统,合理设计来解决这种成骨-血管生成耦合的挑战。该系统在聚乳酸/羟基磷灰石(PLA/HA)支架内集成了结构工程双金属fecu -金属-有机框架(FeCu-MOF)。设计的FeCu-MOF结构可以实现Fe3+和Cu2+离子的程序化和持续的共同释放,提供定制的化学信号。引入协同脉冲电磁场(PEMF)刺激作为物理线索,进一步增强支架的生物活性。由于FeCu-MOF的掺入,复合支架具有相互连接的分层孔隙和增强的亲水性,表现出明显的Fe3+/Cu2+释放谱。在体外,这些支架具有良好的生物相容性,并能显著促进骨髓间充质干细胞(BMSCs)的增殖和成骨分化。值得注意的是,这种结构衍生的双离子释放也显示了促血管生成的潜力。至关重要的是,每日PEMF治疗协同放大了这些细胞反应。在大鼠颅骨缺损模型中的体内评价证实了该系统的有效性。虽然单独使用FeCu-MOF/PLA/HA支架可以促进骨再生,但它们与PEMF的结合效果最显著,其特点是血管化骨形成明显更好。包括显微ct、组织学和免疫组织化学在内的综合分析证实了这些发现,证明了骨体积、密度和结构的改善,成熟的整合组织,以及CD31和成骨标志物耦合表达的增强。总之,该研究验证了增强骨再生的强大协同策略。该策略将可编程的、结构衍生的双金属离子释放与PEMF刺激相结合,成功地实现了协同血管生成-成骨耦合,为复杂缺陷场景提供了一种有前途的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
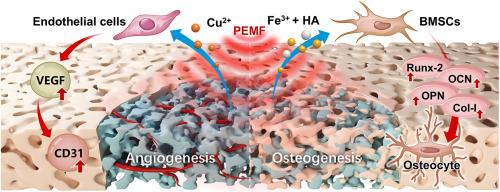
Synergistic dual chemophysical FeCu-MOF scaffold with PEMF stimulation drives angiogenic-osteogenic coupling for bone regeneration
Repairing large bone defects effectively requires concurrent osteogenesis and angiogenesis, a significant challenge for conventional biomaterials often limited by suboptimal structural design and an inability to provide spatiotemporally controlled bioactive cues. Here, we report a novel chemophysical dual-responsive system rationally designed to address this osteogenic-angiogenic coupling challenge. This system integrates a structurally engineered bimetallic FeCu-metal-organic framework (FeCu-MOF) within a poly(lactic acid)/hydroxyapatite (PLA/HA) scaffold. The engineered FeCu-MOF architecture enables the programmed and sustained co-release of Fe3+ and Cu2+ ions, providing tailored chemical signals. Synergistic pulsed electromagnetic field (PEMF) stimulation was introduced as a physical cue to further enhance the scaffold's bioactivity. The composite scaffolds, featuring interconnected hierarchical porosity and enhanced hydrophilicity due to FeCu-MOF incorporation, demonstrated distinct Fe3+/Cu2+ release profiles. In vitro, these scaffolds exhibited excellent biocompatibility and significantly promoted bone marrow mesenchymal stem cells (BMSCs) proliferation and osteogenic differentiation. Notably, this structure-derived dual-ion release also indicated pro-angiogenic potential. Crucially, daily PEMF treatment synergistically amplified these cellular responses. In vivo evaluation in a rat cranial defect model confirmed the system's efficacy. While FeCu-MOF/PLA/HA scaffolds alone enhanced bone regeneration, their combination with PEMF yielded the most robust outcomes, characterized by markedly superior vascularized bone formation. Comprehensive analysis, including micro-CT, histology, and immunohistochemistry, confirmed these findings by demonstrating improved bone volume, density, and architecture, mature integrated tissue, and enhanced coupled expression of CD31 and osteogenic markers. In summary, the study validates a powerful synergistic strategy for enhanced bone regeneration. This strategy, integrating programmable, structure-derived bimetallic ion release with PEMF stimulation, successfully achieved synergistic angiogenic-osteogenic coupling, offering a promising approach for complex defect scenarios.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


